Blood Plasma Partitioning
Knowledge of blood to plasma ratio is important to understanding and predicting the concentration of a drug in blood. Creative Biolabs provides blood to plasma ratio determination service to understand drug distribution in red blood cells and plasma.
The goal of blood plasma partitioning (BPP) is to measure compound concentration ratio between blood and plasma. It is measured by blood concentration ratio/plasma concentration ratio (Cb/Cp) and is an important parameter for predicting whole body pharmacokinetics. Some compounds may have high degree of affinity for the red blood cell fraction of whole blood and so have large blood-to-plasma concentration ratios. For this kind of compounds, PK parameters (e.g. blood clearance) determined by plasma data may be misleading; therefore, PK parameters need to be determined by analysis of whole blood rather than plasma. BPP is usually reflected by red blood cell partitioning (RBC partitioning), which represents the extent of accumulation of a drug in RBC.
To train a drug distribution model, RBC partitioning and hematocrit (HCT) values are required. Test drug is spiked into fresh heparinized whole blood, reference red blood cells and reference plasma. Fractions of each part are analyzed by LC-MS/MS.
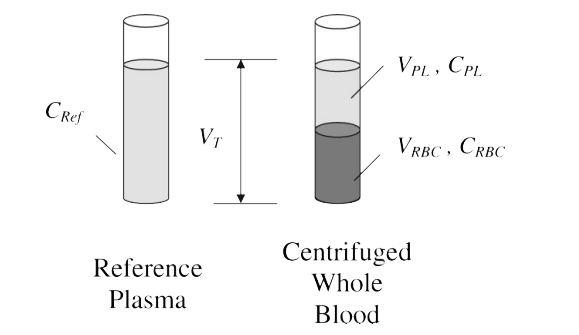 Figure 1. The spiked whole blood sample and the spiked plasma reference sample in the RBC partitioning assay.
Figure 1. The spiked whole blood sample and the spiked plasma reference sample in the RBC partitioning assay.
CRef: reference plasma concentration, which is the same as the concentration of the drug in the whole blood;
CRBC: drug concentration in red blood cells; CPL: drug concentration in equilibrating plasma of the spiked whole blood;
VT, VRBC and VPL: volumes of reference plasma or whole blood, red blood cells, and equilibrating plasma of the whole blood sample, respectively.
A Typical Procedure of BPP Assay
- Fresh heparinized whole blood is stored at 4°C before use.
- A 10 mM stock solution of each compound is prepared in DMSO.
- Reference plasma and whole blood are spiked with tested compound to 5 μM with a final DMSO concentration less than 0.1%.
- Both the spiked whole blood and plasma control are incubated at 37°C for up to 60 min.
- During the time course, aliquots of plasma and whole blood from both incubation mixtures are sampled at 10 and 60 min.
- The whole blood samples are centrifuged to yield the plasma.
- Supernatants from both incubations are extracted using a protein precipitation method, and analyzed using LC/MS/MS.
Determination of the RBC Partitioning Ratio
RBC to plasma partitioning ratio (KRBC/PL) is calculated by the ratio of the concentration of the compound in RBC (CRBC) over that in the equilibrating plasma (CPL). If LC/MS/MS method is used for analysis, the following formula can be used to determine KRBC/PL.
 H: Hematocrit
H: Hematocrit
IPL REF: peak area of the analyte in the reference plasma
IPL: Peak area of the analyte in plasma separated from whole blood
Species Available
We routinely measure and compare KRBC/PL from the following species:
- Mouse, rat, rabbit, dog, monkey, hamster, guinea pig, mini pig and human
For more detailed information, please feel free to contact us or directly sent us an inquiry.
For Research Use Only.
