In Vitro Safety Pharmacology Study on Respiratory System
As a global leader in the area of drug development, Creative Biolabs has accumulated much experience in drug development. In order to optimize leads in pre-clinical drug development, a wide range of in vitro safety pharmacology assays to evaluate the effects of new candidates on CNS system, cardiovascular system, and respiratory system have been established. We try our best to deliver optimum services for global customers at a competitive price.
Safety Pharmacology Core Battery Studies on Respiratory System
The normal function of respiratory system is very vital for maintaining life activities in organism. The regulatory guidelines (ICH S7A/S7B) indicate the core battery of safety pharmacology studies should include respiratory system function studies which are conducted to assess the potential unanticipated adverse effects of compounds on respiratory system. Through these tests, we could have a preliminary understanding of the safety profile of potential new drugs, thus supporting further optimization for the new drugs prior to first administration to humans.
Pumping apparatus and gas exchange unit are two basic functional components of respiratory system. These two components both participate in the regulation of gas exchange between the environment and the airways. The pumping apparatus functions in regulation of gas exchange between environment and airways, ensuring sufficient oxygen is supplied to the circulation system and excess carbon dioxide and other metabolic products are eliminated from the circulation. The gas exchange unit plays a role in regulation gas exchange between airways and blood, ensuring the gas entering the airways is appropriately exchanged with pulmonary arterial blood. In general, respiratory safety pharmacology assays include measures of pumping apparatus and gas exchange unit. Tidal volume, respiratory rate, minute volume, inspiratory, and expiratory and apneic times are the recommended parameters for pumping apparatus measurement. Airway resistance, lung compliance, gas diffusion capacity, and PaO2/PaCO2 are the recommended parameters for gas exchange unit measurement.
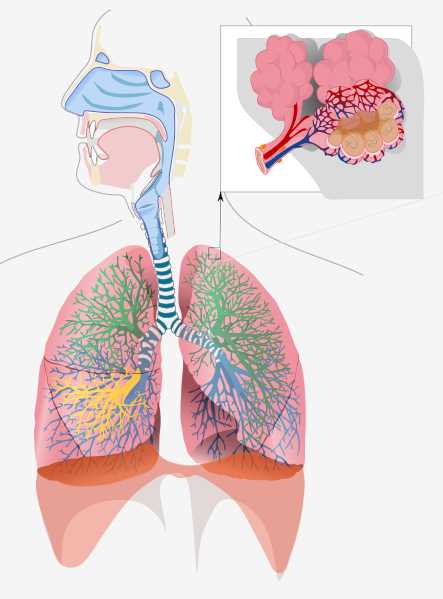 Fig.1 Respiratory system in human body.1
Fig.1 Respiratory system in human body.1
Features
- A full range of solutions for respiratory safety pharmacology
- One-stop service from consultation and project design, to data delivery and interpretation
- Seasoned experts who have engaged in drug development for many years
- Ensured accurate and timely data delivery to accelerate the process of your drug development
Safety pharmacology studies are the key requirement in the preclinical drug development. These studies are conducted to assess the adverse effects of compounds on central nervous, cardiovascular, and respiratory system. With the decades of experience in drug development, Creative Biolabs is capable of offering one-stop superior services covering every aspect of drug development. Respiratory safety pharmacology analysis is one of the most characteristic services. Equipped with excellent expertise and various flexible analytical techniques, we offer unrivaled services for respiratory safety pharmacology studies. If you have a problem with drug development, Please contact us to discuss your project.
Reference
- From Wikipedia: By Bibi Saint-Pol, Jmarchn, CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Respiratory_system_complete_no_labels.svg.
For Research Use Only.
