Infectious Disease Animal Modeling & Pharmacodynamics Services
Introduction
Infectious diseases continue to be a primary contributor to global mortality, causing over 13 million deaths annually and accounting for more than 25% of all human fatalities. Although there have been advances in worldwide prevention and control measures, the complete eradication of these diseases continues to be a daunting challenge. Creative Biolabs offers comprehensive model system, a wide spectrum of pathogen, validated models, reliable results and full-cycle infectious disease model services, offering customized animal models (including rodents, non-rodents, non-human primates, fruit flies, and zebrafish) and cellular models tailored to study viruses, bacteria, fungi, and parasites. Our advanced models facilitate the acceleration of drug R&D and the Investigational New Drug (IND).
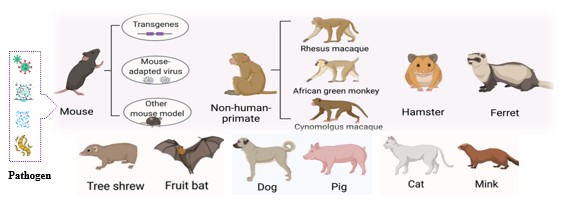 Fig. 1 Animal species of animal models for infectious diseases.1,3
Fig. 1 Animal species of animal models for infectious diseases.1,3
Available Infectious Disease Models
Creative Biolabs can facilitate a variety of pre-clinical R&D, including vaccines, chemical drugs, gene therapies, biological drugs, cell therapies and novel therapeutic approaches. Learn more about the existing well-characterized models of infectious diseases models for preclinical research by clicking on the links below:
Available Pathogens
Pathogen reservoirs utilized in our infectious disease models encompass
| Classify | Pathogen type | |
Viral 
|
Respirovirus | Influenza virus A/B/C, SARS-CoV-2, Respiratory syncytial virus (RSV), Adenovirus, Rhinovirus, Measles virus, Mumps virus, Varicella zoster virus |
| Gastrointestinal tract viruses | HAV, HEV, Rotavirus, Norovirus | |
| Blood/STIs viruses | Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human immunodeficiency virus (HIV)-1/-2, Human papillomavirus (HPV, more than 100 types), Herpes simplex virus (HSV)-1/-2, Epstein-Barr virus | |
| Arbovirus | Dengue virus, Zika virus, Yellow fever virus, Japanese encephalitis virus | |
| Zoonotic viruses | Rabies virus, Hantavirus, Monkeypox virus, Ebola virus | |
Bacterial
|
Gram-positive | Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, Enterococcus faecalis, Bacillus anthracis), Clostridium tetani, Clostridium perfringens, etc. |
| Gram-negative | Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus influenzae, Escherichia coli, Salmonella typhi, Shigella, Pseudomonas aeruginosa, Klebsiella pneumoniae, Helicobacter pylori, Brucella, Yersinia pestis, Legionella pneumophila, etc. | |
| Others | Mycobacterium tuberculosis, Mycobacterium leprae, Bordetella pertussis, Campylobacte, Treponema pallidum, Leptospira spp., Rickettsia spp., Rickettsia spp., etc. | |
|
Fungal |
Yeast fungi | Candida albicans, Candida tropicalis, Cryptococcus neoformans, Penicillium marneffei |
| Filamentous fungi | Aspergillus fumigatus, Aspergillus flavus, Mucor spp., Rhizopus spp., Sporothrix schenckii | |
| Skin fungi | Dermatophytes, Trichophyton spp., Microsporum spp., Epidermophyton spp. | |
| Deep fungi | Histoplasma capsulatum, Coccidioides immitis, Paracoccidioides brasiliensis, Blastomyces dermatitidis | |
|
Parasitic |
Protozoon | Plasmodium spp., Toxoplasma gondii, Entamoeba histolytica, Giardia lamblia, Trichomonas vaginalis, Leishmania donovani, Trypanosoma brucei, Trypanosoma cruzi, etc. |
| Fluke | Schistosoma japonicum, Schistosoma mansoni, Clonorchis sinensis, Paragonimus westermani | |
| Tapeworm | Taenia solium, Taenia saginata, Echinococcus granulosus | |
| Nematode | Ascaris lumbricoides, Ancylostoma duodenale, Necator americanus, Enterobius vermicularis, Trichuris trichiura, Wuchereria bancrofti, Brugia malayi | |
Measurements
Pharmacodynamic models are subjected to rigorous validation, employing multi-modal analytical platforms to ensure their accuracy and reliability.
- Clinical Monitoring: Systematic evaluation of biometric parameters (coat and skin condition, body weights, organ indices), physiological status (respiratory function, mental status), behavioral metrics (locomotor activity, mental status), and survival outcomes.
- Virological Profiling: Viral quantification via TCID50 assays, plaque assays, and antigen detection through ELISA/immunofluorescence.
- Immune Response Mapping: Multiplex cytokine analysis across biological matrices, complemented by flow cytometric immunophenotyping.
- Molecular Biology: Transcript-level verification through quantitative reverse transcription PCR (RT-PCR) and protein expression validation via immunoblotting.
- Systemic Pathological Evaluation: Consolidated hematology, serum biochemistry, advanced histopathology, and micro-CT imaging.
Related Disease Models
Inflammation & Immunological Disease Models
Metabolic/Liver Disease Models
Urological System Disease Models
Musculoskeletal Disease Models
Ear Disorder Models
Reproductive System Disease Models
Skin Disease Mode
Our Advantages
- Comprehensive Service Chain: Our integrated service chain encompasses the entire drug development lifecycle, from target discovery and preclinical research through IND application support.
- Multidisciplinary Team Composition: Our team consists of multidisciplinary experts who provide professional, customized, and precise research programs for infectious animal models, thereby expediting project advancement and reducing the new drug development cycle.
- Data and Experience Accumulation: A continuously expanding database of standardized preclinical models and a comprehensive pathogen library. This extensive resource facilitates the rapid deployment of comparative approaches for various therapeutic programs.
- One-stop Services: Multidisciplinary teams provide bespoke modeling strategies, leveraging expertise in virology, immunology, and translational medicine to satisfy IND requirements.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q. What types of pathogens can Creative Biolabs’ infectious animal models accommodate?
A: Creative Biolabs' infectious animal models can accommodate a broad spectrum of pathogens, including viral agents, fungal species, and worms. For pathogens not explicitly listed, proprietary model development services are available upon consultation.
-
Q. How does Creative Biolabs achieve reproducibility in infectious disease animal models?
A: Strict standardization governs pathogen preparation, animal husbandry (SPF conditions), challenge protocols (dose-response validation), and endpoint analysis. All experimental participants have received professional training, and we use validated models verified by QA and QC during the experiment to ensure data authenticity and experimental repeatability.
-
Q. Does Creative Biolabs offer customized infectious model development?
A: Certainly. Creative Biolabs not only offers verified models, but also provides custom model development and validation services, specifically tailored to meet your unique requirements.
-
Q: What are the key advantages of choosing Creative Biolabs’ infectious model services?
A: Opting for Creative Biolabs' infectious model services, as opposed to establishing in-house animal models, presents several key benefits:
Established Expertise: Access to mature animal modeling experience and specialized animal facilities.
Expert Personnel: Engagement with a team of experienced scientists.
Advanced Equipment: Utilization of cutting-edge experimental equipment.
Reduced Complexity: Streamlined processes minimize procedural complexities.
Our streamlined processes minimize procedural complexities, leading to a significant reduction in both time and expenses associated with personnel training. This ultimately expedites research and development timelines.
-
Q: What will you gain when Creative Biolabs completes the entire Pharmacodynamics infectious model R & D process?
A: Upon the completion of the research and development process by Creative Biolabs, the recipient will receive a comprehensive evaluation report. This report will encompass tailored methodologies, professionally analyzed results, and the accompanying raw data.
Published Data
Mouse Cholera Model: To evaluate the efficacy of combined oral and parenteral cholera vaccina. Fecal Bacterial Colonization indicate transient intestinal colonization by the vaccine strain (Fig. 16A), the combination group (oral and injectable) exhibited the highest vibriocidal antibody titers and OSP-Specific IgM Memory B Cell Responses (P < 0.05, Fig. 12B-C).
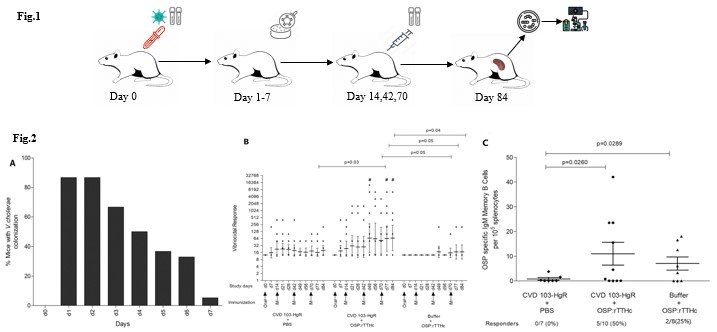 Fig.2 The efficacy of combined oral and parenteral cholera vaccination.2,3
Fig.2 The efficacy of combined oral and parenteral cholera vaccination.2,3
References
- Bi, Zhenfei, Hong Weiqi, Yang Jingyun, et al. Animal models for SARS-CoV-2 infection and pathology. MedComm 2.4 (2021): 548-568. https://doi.org/10.1002/mco2.98
- Akter, Aklima, Meagan Kelly, Charles Richelle C, et al. Parenteral vaccination with a cholera conjugate vaccine boosts vibriocidal and anti-OSP responses in mice previously immunized with an oral cholera vaccine. The American Journal of Tropical Medicine and Hygiene 104.6 (2021): 2024. https://doi.org/10.4269/ajtmh.20-1511
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.