What is Glycosylation and Why is Glycosylation Important?
What is Glycosylation?
Glycosylation is the combination of a carbohydrate with a hydroxyl or other functional group of another molecule to form a glycoconjugate. It is a form of co-translation and post-translation modification. Most proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as O-GlcNAC modification.
Main Types of Glycosylation in Humans
Many proteins are modified by N-glycosylation, which refers to the attachment of N-acetylglucosamine (GlcNAc) to the nitrogen atom of an Asn side chain by α β-1N linkage. These Asn-linked glycoconjugates contain a GlcNAc2 mannose (Man)3 core, to which a variable number of other monosaccharides can be added or removed. These additions include, for example, galactosylation, GlcNAclyation, sialylation, and fucosylation, and they determine whether the final structure is classed as a high-mannose N-glycan, a hybrid N-glycan, or a complex N-glycan. N-glycosylation depends on the formation of a lipid precursor.
Glycosylation can occur on amino acids with functional hydroxyl groups, which are most often Ser and Thr. In humans, the most common sugars linked to Ser or Thr are GlcNAc and N-acetylgalactosamine (GalNAc). GalNAc-linked glycans, often called mucin-type O-glycans, are abundant on many extracellular and secreted glycoproteins. Mucin-type O-glycan synthesis is initiated by polypeptide GalNAc transferases (GALNTs). Moreover, O-GlcNAc modification competes with protein phosphorylation at Ser and Thr residues, adding to the complexity of regulatory circuits. This functional role of O-glycosylation can be controlled by the expression levels of the enzymes involved, as well as substrate concentrations.
-
Glycosphingolipids (GSLs)
GSL glycosylation starts with the addition of glucose or galactose to the lipid moiety at the cytoplasmic side of the ER or the Golgi apparatus, but the structure is then flipped to the luminal side for further processing. The enzymes that initiate GSL glycosylation are specific for lipids, but further processing of the carbohydrate chain can be performed by more general glycosyltransferases.
-
Proteoglycans and Glycosaminoglycans
Proteoglycans are glycoproteins in the extracellular matrix that, in addition to containing canonical N-glycans and O-glycans, are characterized by the presence of long sugar repeats attached via O-linked glycosylation motifs. These extended sugar chains are termed glycosaminoglycans and contribute to a substantial proportion of the proteoglycan’s molecular mass. Whereas N-glycans typically include 5-12 monosaccharides, a glycosaminoglycan motif can easily contain more than 80 sugars.
-
Extrinsic Glycosylation Events
Extrinsic glycosylation is the process by which soluble glycan-modifying enzymes, such as glycosyltransferases that circulate in the blood, conjugate a monosaccharide to an existing sugar structure extracellularly. Common acceptor structures for extrinsic glycosylation on mammalian glycoconjugates are galactose (Gal)(β3)-GlcNAc, Gal(β3)-GalNAc, Gal(β4)-GlcNAc and GalNAc; β3 and β4 represent two different linkage positions on the conjugating sugar.
Diseases of Glycosylation
Defects in the anabolic process of glycan formation are more typically considered human diseases of glycosylation. Inclusion cell disease was the first to be identified and was shown to result from failure to produce the mannose 6-phosphate modification on N-glycans in the Golgi. An increasing number of diseases of glycosylation are being discovered, especially in the pediatric clinic among children during the first few years of life. Human glycosylation disorders identified so far are primarily severe syndromes, many of which reflect the disruption of early steps in the pathways of glycan biosynthesis. Diseases that alter glycosylation have revealed new mechanisms regarding how glycan formation is regulated. A recent analysis of glycoprotein mutations in the human disease database revealed a larger-than-random occurrence of amino acid changes in proteins that predict a gain-of-N-glycosylation, and those that were tested indeed acquired an N-glycan concurrent with glycoprotein dysfunction.
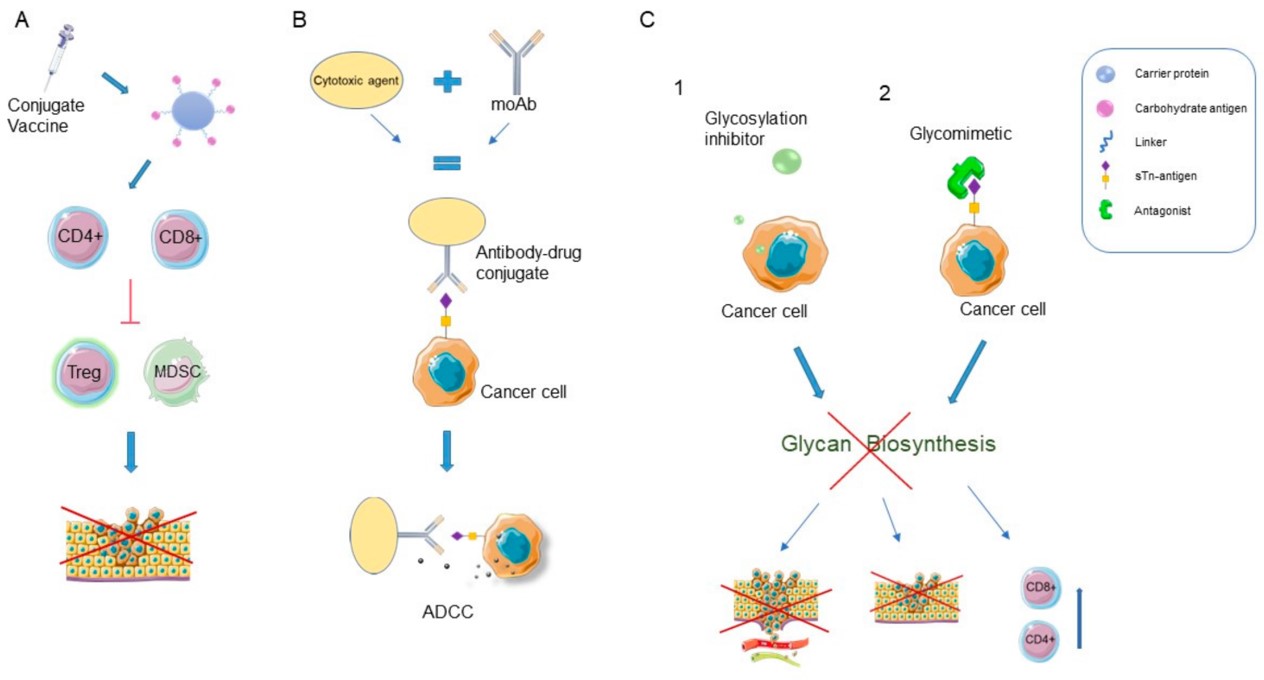 Fig.1 Glycan-based cancer therapies.1, 2
Fig.1 Glycan-based cancer therapies.1, 2
Services and Technologies for Glycosylation Research at Creative Biolabs
Custom Services for Glycobiology Research
Creative Biolabs focuses on glycosylation research for years. Our senior experts and rich projects experience make us an excellent service provider and problem solver. With the strong technology platform, we provide custom services for you to advance your projects. If you are interested in our service, please contact us for more information.
References
-
Peric, Luka, et al. "Glycosylation alterations in cancer cells, prognostic value of glycan biomarkers and their potential as novel therapeutic targets in breast cancer." Biomedicines 10.12 (2022): 3265.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Glycan-based cancer therapies.1, 2
Fig.1 Glycan-based cancer therapies.1, 2

