What Are Carbohydrate Chains and Glycans?
When we think about the molecules that shape our cells, we often focus on proteins, DNA, and lipids. However, carbohydrate chains, or glycans, are just as essential. These sugar molecules are not just attached to proteins and lipids—they actually help drive a variety of key processes in our bodies. From enabling immune cells to recognize invaders to helping cells communicate with each other, glycans are integral to health. A glycan is a chain of sugar units that can be attached to proteins (called glycoproteins) or lipids (called glycolipids). Their structure can vary widely, depending on the sugars involved and how they are linked together. These sugars don't just serve as structural elements—they also play a crucial role in disease, particularly when the balance of glycosylation (the process of adding sugars to molecules) is disrupted. This change can lead to a range of conditions, from cancer to autoimmune disorders and infections.
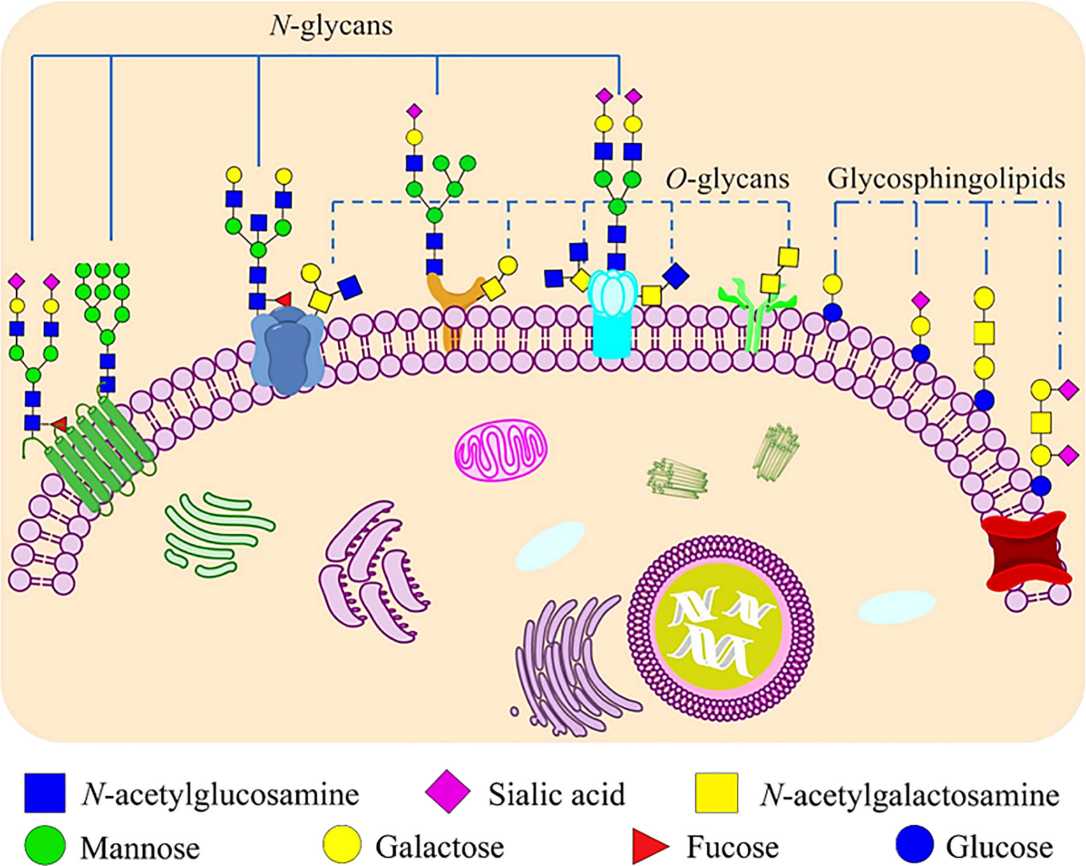 Fig.1 Glycoconjugates that formed by carbohydrates are covalently bonded to proteins and lipids on mammalian cell membranes.1,3
Fig.1 Glycoconjugates that formed by carbohydrates are covalently bonded to proteins and lipids on mammalian cell membranes.1,3
Why Glycobiology Matters in Disease?
The field of glycobiology, which focuses on understanding how glycans influence cell biology, is gaining more attention as it becomes clear how important these sugar chains are in disease. In healthy cells, glycosylation is tightly regulated, making sure that the right sugars are added at the right time. But when this regulation goes awry, it can lead to abnormal cell behavior, such as tumor growth or immune system dysfunction. For example, cancer cells often have altered glycans on their surfaces, allowing them to evade detection by the immune system and metastasize to other parts of the body. Similarly, in autoimmune diseases, glycosylation changes can cause the immune system to attack the body's own cells. Glycosylation in disease is now recognized as a major factor in the progression of many diseases, and understanding how it works could unlock new ways to treat these conditions.
Glycosylation in Disease: Mechanisms and Implications
Disrupted Glycosylation in Inflammatory and Autoimmune Diseases
Glycosylation abnormalities have been implicated in various autoimmune disorders, including rheumatoid arthritis (RA), lupus, and multiple sclerosis (MS), where they influence both immune signaling and tissue recognition. In RA, altered glycosylation of immunoglobulin G (IgG) can lead to the production of autoantibodies, exacerbating inflammation by forming immune complexes that are misinterpreted as foreign pathogens by immune cells. Studies show that IgG in RA patients exhibits significant changes in glycosylation, including an increased presence of galactose-deficient oligosaccharides, which promotes inflammation. Similarly, in MS, disrupted glycosylation of T-cell receptors affects their interaction with self-antigens, making these cells more likely to attack the central nervous system. This dysfunction in glycosylation complicates therapeutic strategies, as altering the glycosylation of therapeutic proteins, such as monoclonal antibodies, can change their efficacy by affecting their ability to interact with immune cells.
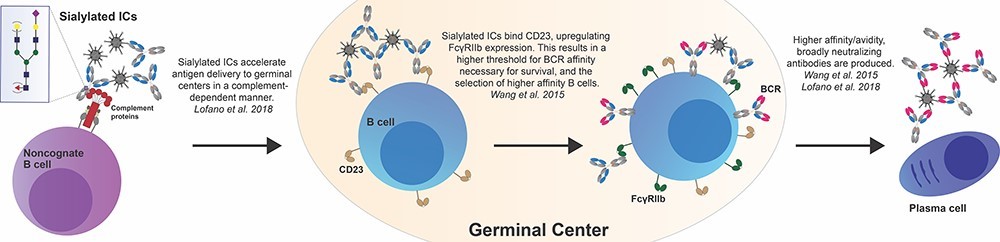 Fig.2 Potential roles for IgG Fc sialylation in driving the evolution of higher avidity and affinity antibody responses.2,3
Fig.2 Potential roles for IgG Fc sialylation in driving the evolution of higher avidity and affinity antibody responses.2,3
Glycosylation Modifications in Infectious Diseases and Pathogen Recognition
In infectious diseases, pathogens exploit the host's glycosylation machinery to invade cells and evade immune detection. For instance, both the influenza virus and HIV are heavily reliant on glycosylation to bind to host cell receptors. The influenza hemagglutinin (HA) glycoprotein, for example, uses sialic acid (SA) residues for binding to host cell receptors. Disruptions in the glycosylation patterns of host cell receptors can alter the host's ability to recognize these pathogens, potentially leading to either immune evasion or excessive inflammation. The HIV glycoprotein gp120, for example, uses N-linked glycans to shield its antigenic sites, allowing the virus to evade neutralizing antibodies. This glycan shielding plays a crucial role in immune escape and is a significant challenge for vaccine development.
Altered Glycosylation in Cancer Cells: A Key to Tumor Development and Metastasis
In cancer, altered glycosylation contributes to tumor progression and metastasis. Cancer cells often exhibit a shift in glycosylation patterns, such as increased levels of sialylation and fucosylation, which enhance cell migration and immune evasion. These glycosylation modifications are crucial in regulating cell adhesion and detachment, key processes for metastasis. For example, the overexpression of fucosylated glycans on the surface of tumor cells aids in detachment from the primary tumor and spread to distant organs. Additionally, these altered glycans can modulate immune recognition, either hiding tumor-associated antigens from immune surveillance or inhibiting the immune response. Understanding these changes has led to the exploration of glycosylation-targeted therapies in cancer treatment, which seek to block these pathways to inhibit tumor growth and metastasis.
Carbohydrate Chains in Cancers
Cancer cells are like imposters that our bodies often fail to recognize as foreign. But these cells have a unique biochemical signature: they often display specific sugar molecules on their surface that normal cells don't. By targeting these unique glycans, we can create highly specific glycan-targeted immunotherapies. For instance, we can develop monoclonal antibodies that latch onto these sugary markers, acting as a beacon for our immune system. This approach not only helps our immune cells recognize and attack cancer cells but also enhances the effectiveness of other cancer treatments, making it a promising avenue for personalized medicine. Furthermore, by modifying the unique sugar coatings, or glycosylation patterns, on cancer cells, we can potentially enhance the effectiveness of other cancer treatments, such as immunotherapy. This opens up exciting possibilities for personalized medicine by tailoring therapies to the specific sugar signatures of individual tumors.
Carbohydrate Chain Biomarkers for Early Detection and Prognosis
Early cancer detection is crucial for boosting survival rates, and one of the most promising avenues for achieving this lies in identifying changes in glycosylation patterns. In essence, the carbohydrate chains (glycans) on cells often undergo alterations during the early stages of tumor development. These changes aren't confined to the tumor itself; they can be detected in readily accessible bodily fluids like blood, urine, and even saliva. Pinpointing these alterations early offers a significant advantage: it can enable cancer diagnosis before any noticeable symptoms arise, dramatically improving treatment outcomes.
Beyond early detection, these glycosylation markers also hold predictive power regarding disease progression. Certain glycan patterns, for example, are linked to more aggressive cancer types. By keeping track of these markers, doctors gain valuable insights into likely patient outcomes, allowing them to personalize treatment strategies more effectively. Below, you'll find a table summarizing some key carbohydrate chain biomarkers associated with different types of cancer. We also provide Oligosaccharide Library Development Service to support your biomarker discovery efforts by creating custom oligosaccharide libraries.
|
Cancer Type
|
Glycan Biomarker
|
Clinical Application
|
|
Breast Cancer
|
MUC1 glycoprotein, sialylated structures
|
Early detection via blood tests
|
|
Colorectal Cancer
|
Tn-antigen, sialylated T-antigen
|
Prognostic marker, predictive of metastasis
|
|
Lung Cancer
|
Fucose and galactose-containing glycoproteins
|
Monitoring tumor progression and treatment response
|
|
Pancreatic Cancer
|
CA19-9 glycan antigen
|
Diagnostic marker for early-stage detection
|
Glycan-Targeted Drug Delivery Systems and Nanomedicine
Glycan-targeted drug delivery is transforming disease treatment, especially in cancer. Instead of spreading drugs throughout the body, this approach directs therapies precisely to cells with specific carbohydrate structures. By focusing treatment on diseased cells, it minimizes harm to healthy tissues. Nanomedicine plays a key role here—tiny nanoparticles can carry drugs and are designed to bind to glycans on cancer or infected cells. This targeted delivery boosts treatment effectiveness while reducing side effects, offering new possibilities for treating cancers, infections, and other diseases.
Advanced Glycomics for Identifying Disease-Specific Glycan Signatures
With advances in glycomics, scientists can now identify disease-specific glycan patterns using techniques like mass spectrometry and glycan microarrays. These tools reveal how glycan structures on cell surfaces change in disease states, helping us understand how glycosylation influences diseases like cancer and autoimmune disorders. By pinpointing these changes, researchers can develop more precise diagnostic tests and therapies that target the glycans driving disease. This is a crucial step toward personalized medicine, where treatments are tailored to an individual's unique disease profile.
Understanding carbohydrate chains in disease is unlocking new opportunities for treatments. Glycosylation plays a critical role in disease progression, and targeting these sugar molecules opens the door to more effective, personalized therapies. Creative Biolabs is leading the way in facilitating your research in innovative therapies that directly target glycans.
References
-
Irvine, Edward B., and Galit Alter. "Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases." Glycobiology 30.4 (2020): 241-253.
-
Li, Yuqing, et al. "The importance of glycans of viral and host proteins in enveloped virus infection." Frontiers in Immunology 12 (2021): 638573.
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Glycoconjugates that formed by carbohydrates are covalently bonded to proteins and lipids on mammalian cell membranes.1,3
Fig.1 Glycoconjugates that formed by carbohydrates are covalently bonded to proteins and lipids on mammalian cell membranes.1,3
 Fig.2 Potential roles for IgG Fc sialylation in driving the evolution of higher avidity and affinity antibody responses.2,3
Fig.2 Potential roles for IgG Fc sialylation in driving the evolution of higher avidity and affinity antibody responses.2,3

