O-Linked Glycoengineering Service in Yeast
O-Glycosylation in Yeast
There are differences in the synthetic pathway between yeast O-glycans and the main human-type O-glycans. Mucin-type glycosylation, the most prevalent form of O-glycosylation in mammals, is initiated with the attachment of a GalNAc residue to specific Ser/Thr residues. Subsequent elongation with various monosaccharides leads to the formation of a highly diverse array of oligosaccharides, which is orchestrated by GalNAc transferases in the Golgi apparatus, utilizing UDP-GalNAc as the donor.
Differently, yeasts perform O-glycosylation by attaching oligomannosyl-glycans to proteins. The initiating glycosyltransferases responsible for yeast O-mannosyl glycans belong to a highly redundant enzyme family, protein-O-mannosyltransferases (PMTs). These enzymes transfer a single mannose from dolichol-P-mannose to Ser or Thr residues. Subsequently, additional α-mannose residues are added to the glycan by various mannosyltransferases in the Golgi employing GDP-mannose as the donor substrate. As yeast-type O-glycans differ structurally from human O-glycans, concerns have arisen regarding their potential immunogenicity in the development of biotherapeutics. Consequently, yeast O-glycosylation has emerged as an engineering target.
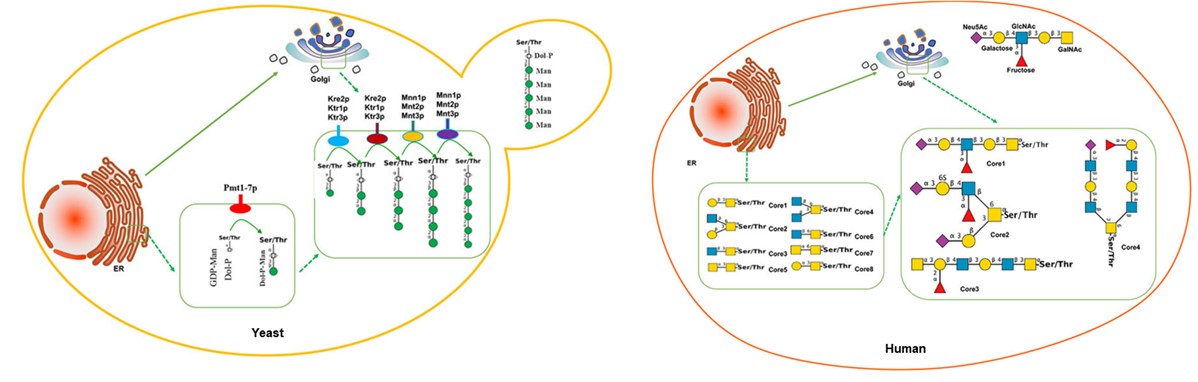 Fig.1 O-glycosylation pathway in yeast and human cells.1, 2
Fig.1 O-glycosylation pathway in yeast and human cells.1, 2
O-Linked Glycoengineering Services in Yeast at Creative Biolabs
Creative Biolabs has successfully introduced several human-type O-glycosylation pathways into yeast based on multiple glycoengineering strategies. Leveraging our comprehensive understanding of glycosylation mechanisms in both yeast and humans, we employ various genetic interventions, including Knockout, Knockin, and Overexpression techniques, to achieve these modifications. Since yeasts exclusively perform O-mannosylation through PMTs when expressing human O-glycoproteins, we employ different strategies to block the initiation of O-mannosylation. Following the suppression of native O-mannosylation, we introduce the entire biosynthetic machinery required for synthesizing and transporting human O-glycans into yeast. This engineered and customized pathway is designed to enable the production of a variety of human-type O-glycans, making yeast a versatile platform for various glycoengineering applications.
Removing or reducing native O-glycans in yeast
-
Eliminating the PMT family of proteins by gene knockout.
-
Reducing PMT activities with chemical inhibitors such as R3A.
-
Enzymatic trimming of O-mannosyl through expression of mannosidases.
Engineering human-type O-glycans in yeast
-
Engineering of O-fucosylation.
-
Engineering of mucin-type glycosylation.
-
Engineering of α-dystroglycan-type glycosylation.
Features of Our Services
-
Multi-targeted and optional glycoengineering strategies
-
Highly efficient and precise knockin or knockout
-
High-quality glycoprotein production with fully humanized O-glycans
-
Customized glycoengineering for specific needs
Multiple glycoengineering strategies have been devised to either eliminate yeast-specific glycans from recombinant glycoproteins or transform them into human-type O-glycans. Creative Biolabs is committed to providing these glycoengineering services to help our clients with their specific needs. Please don't hesitate to contact us for more information.
References
-
Li, Xingjuan, et al. "Humanization of yeasts for glycan-type end-products." Frontiers in Microbiology 13 (2022): 930658.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 O-glycosylation pathway in yeast and human cells.1, 2
Fig.1 O-glycosylation pathway in yeast and human cells.1, 2

