N-glycans
Introduction of N-glycans
The first structures of N-glycans were determined in the 1970s using a combination of biochemical strategies to identify the sugars attached to Asn in certain glycoproteins, and their linkage arrangements. The N-glycans could be divided into three types: complex, hybrid and high mannose N-glycans. The clue to their origin was that each N-glycan type contains the same core region consisting of two GlcNAc and three Man residues.
The synthesis of N-glycans is a complicated pathway involving all the basic building blocks of the cell - lipids, proteins, nucleic acids, and sugars. Therefore, defects in many different aspects of cell metabolism may cause the altered synthesis of N-glycans, which may in turn lead to human disease. A large group of diseases included in the Congenital Disorders of Glycosylation (CDG) has been shown to arise from defective N-glycan synthesis.
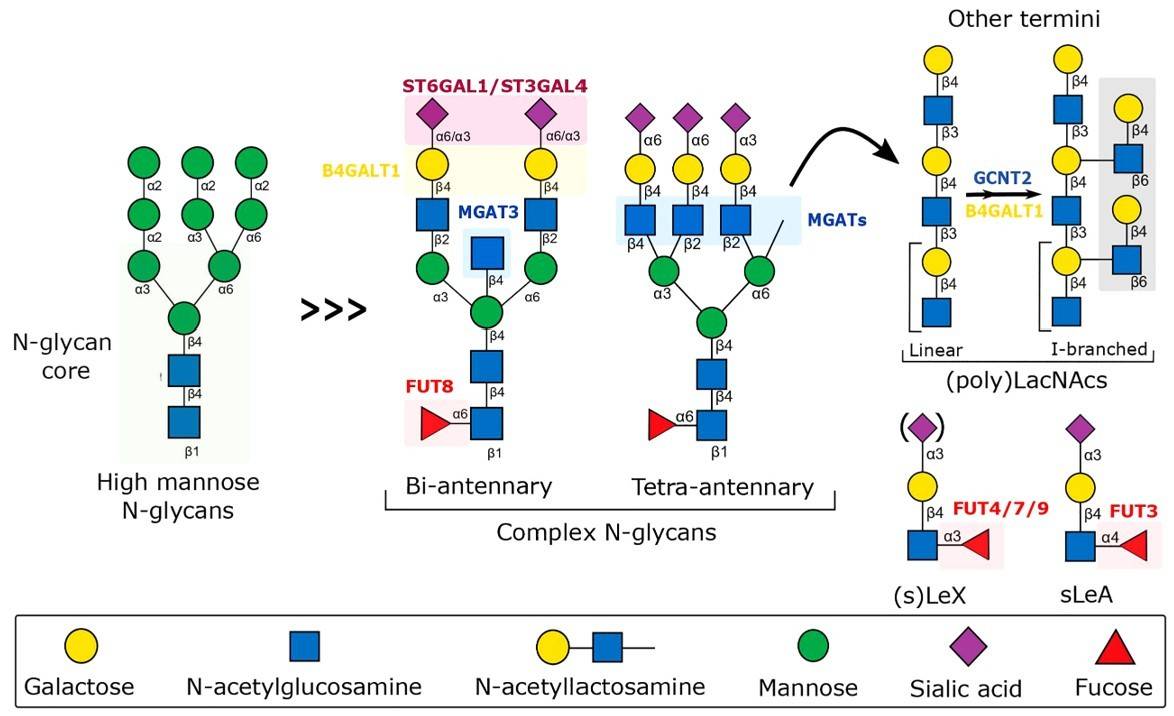 Fig.1 Structure and biosynthesis of N-glycans.1, 2
Fig.1 Structure and biosynthesis of N-glycans.1, 2
Functions of N-Glycans
-
Physical properties of a glycoprotein
N-Glycans contribute to the physical properties of a glycoprotein in terms of its solubility, resistance to proteases and half-life, particular N-glycan residues, or groups interactions with glycan-binding proteins that regulate the expression, activity and function of a glycoprotein.
-
Chaperones Recognize High Mannose N-Glycans on Unfolded Glycoproteins
In the endoplasmic reticulum (ER), chaperones and enzymes that bind to specific regions of high mannose N-glycans lead to optimal glycoprotein folding, or degradation by ER-associated degradation, or ubiquitination and translocation to the cytosol and degradation by the proteasome.
Lysosomal hyrolases destined to reside in lysosomes where they degrade proteins, lipids and carbohydrates are escorted through the Golgi compartments and into endosomes by Man-6-P receptors which recognize Man-6-P exposed following removal of the GlcNAc transferred by phosphoGlcNAc-transferase.
Functions of Complex N-Glycans at the Cell Surface
N-glycans are the primary ligands for mammalian lectins such as the galectins that recognize Gal residues Siglecs bind to sialic acid residues and selectins bind to SLeX. Pathogens such as the influenza virus bind to sialic acid, and cell-cell interactions in the nervous system are mediated by polysialic acid on N-CAM. In addition, studies have shown that the branching of complex N-glycans and the length of polylactosamine on each branch dictates the nature and strength of galectin interactions with N-glycans attached to signaling receptors.
Complex N-glycans may be used to target therapeutic glycoproteins to cells with specific glycan-binding receptors. For example, the removal of sialic acid to expose terminal Gal residues causes glycoproteins to be rapidly cleared from the circulation by the Ashwell-Morrell receptor in the liver. This may be used as a strategy for targeting therapeutics to the liver.
Services at Creative Biolabs
N-linked glycosylation is the most important glycosylation modification due to its significance in modulating the stability, function and structural integrity of biopharmaceuticals. Inspection of the protein databases suggests that as many as 70% of proteins have potential N-glycosylation sites. Modulation of N-glycosylation during recombinant engineering in the pharmaceutical industry is challenging. The N-glycosylation profile of recombinant glycoprotein depends on several culture conditions, including nutrient levels, dissolved oxygen level, pH, temperature, high stress, use of serum, growth on microcarriers and media additives.
Creative Biolabs has a number of experts and a complete equipment platform in the field of glycoproteins. With unique advantages, we have mastered several important control points in the production process of recombinant glycoproteins. We are confident to provide you with the best custom glycan synthesis service. If you have any questions, please contact us for more information.
References
-
Radovani, Barbara, and Ivan Gudelj. "N-glycosylation and inflammation; the not-so-sweet relation." Frontiers in immunology 13 (2022): 893365.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Structure and biosynthesis of N-glycans.1, 2
Fig.1 Structure and biosynthesis of N-glycans.1, 2

