O-glycans
Introduction of O-glycans
O-linked glycosylation refers to the attachment of a sugar molecule to the peptide chain through an oxygen atom of serine (Ser) or threonine (Thr) residues. In addition, Tyrosine (Tyr), hydroxylysine (Hydroxy-Lys), or hydroxyproline (Hydroxy-Pro) might be also a peptide site of O-linked glycosylation. O-glycosylation occurs widely in all areas of life, such as eukaryotes, archaea, and pathogenic bacteria. Changes in O-glycosylation are important for a variety of diseases, including cancer, diabetes, and Alzheimer's disease.
The mucin-type glycans, which contain an initial GalNAc residue, are the most common O-linked glycans. And there are four major core structures for the O-GalNAc glycans of mucins. These core structures can be extended, branched, and terminated with Fuc, Sia, or blood group antigenic determinants.
Common Types of O-glycosylation
-
O-N-acetylgalactosamine (O-GalNAc)
Under the action of GalNAc transferase (GALNT), the addition of N-acetylgalactosamine (GalNAc) and serine or threonine occurs in the Golgi apparatus. The original O-GalNAc structure can be modified by adding other sugars or other compounds to produce the known 8 core structures.
-
O-N-acetylglucosamine (O-GlcNAc)
As the first glycosylation that does not occur on secreted proteins, the addition of N-acetylglucosamine (O-GlcNAc) with serine or threonine usually occurs on cytoplasm and nuclear proteins.
O-mannosylation refers to the process that mannose is transferred from the dolichol-P-mannose donor molecule to the serine or threonine residues of the protein. This process is initiated on the endoplasmic reticulum of the cell.
O-galactose is usually present on the lysine residues of collagen, and a hydroxyl group is usually added to form hydroxylysine.
O-Fucosylation is the process of adding fucose to serine and threonine residues under the catalysis of two fucosyltransferases. This process occurs in the endoplasmic reticulum.
Similar to O-fucosylation, O-glucosylation is an unusual O-linked modification that occurs in the endoplasmic reticulum and is catalyzed by O-glucosyltransferase.
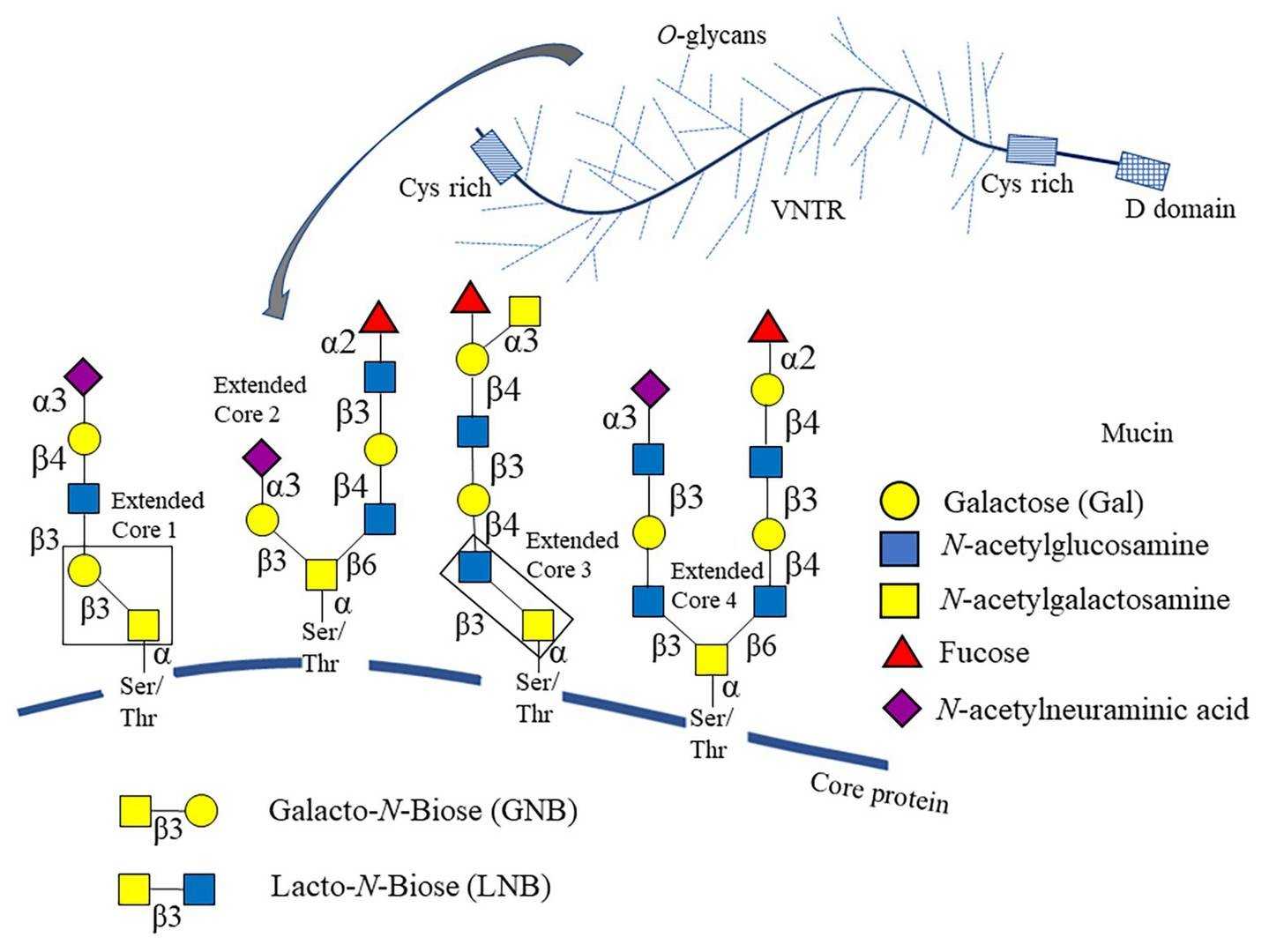 Fig.1 O-glycan cores linked to mucin.1, 2
Fig.1 O-glycan cores linked to mucin.1, 2
Functions of O-glycans
-
Trafficking of cells in the immune system
-
Control cell metabolism
-
Allow recognition of foreign material
-
Provide cartilage and tendon flexibility
-
Leukocyte circulation during an immune response
Methods for Characterization of O-Glycans
-
Liquid chromatography
-
Exoglycosidase arrays
-
Mass spectrometry techniques
-
Fragmentation approaches
-
Capillary electrophoresis - A well-established method for the separation and analysis of O-glycans.
-
Nuclear magnetic resonance - Has been used for the structural determination of O-glycans.
Creative Biolabs has been a long-term expert in the field of glycomics. As a pioneer and the undisrupted global leader in glycan research, we offer a variety of products and services including custom glycan synthesis. If you are interested in our products or services, please do not hesitate to contact us for more detailed information.
References
-
González-Morelo, Kevin J., Marco Vega-Sagardía, and Daniel Garrido. "Molecular insights into O-linked glycan utilization by gut microbes." Frontiers in microbiology 11 (2020): 591568.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 O-glycan cores linked to mucin.1, 2
Fig.1 O-glycan cores linked to mucin.1, 2

