Biosynthesis of GPI Membrane Anchors
Overview of GPI Membrane Anchors
The glycosylphosphatidylinositol (GPI)-anchored proteins (GPI-APs) are a class of proteins, expressed from the protozoa, fungi, to humans, representing 0.5% of total proteins in eukaryotes. Interestingly, approximately 1% of plant proteins appear to be modified with a GPI anchor, and, as in mammals, the loss of GPI-anchoring results in lethality. To date, plant GPI-APs have been found to play critical roles at the interface of the plasma membrane and cell wall where they are mostly localized. More than 150 GPIAPs have been identified in mammalian cells and they exhibit diverse functions including surface enzymes, antigens, receptors, cell adhesion molecules, and complement regulatory components.
Many eukaryotic cell proteins are anchored to membranes by covalent linkage to glycosylphosphatidylinositol. In humans, more than 150 proteins are membrane-anchored via a GPI, as highlighted by different technical approaches and membrane fractions. Moreover, hundreds of GPI-APs are potentially encoded by the human genome. They perform or mediate various critical cellular functions, including signal transduction, cell adhesion, and immune recognition.
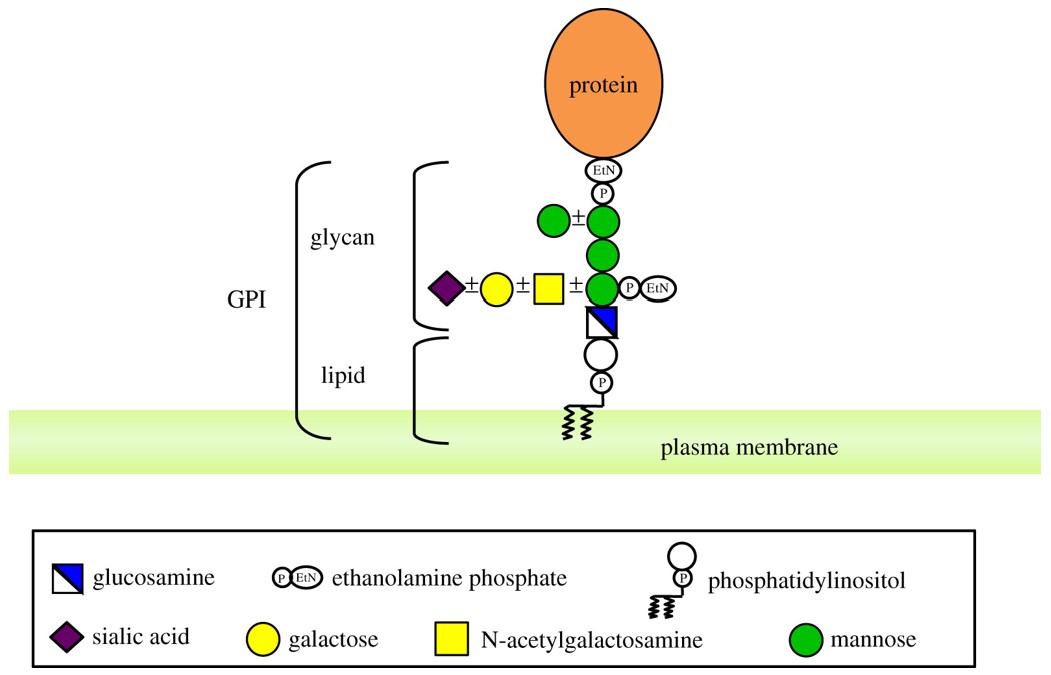 Fig.1 GPI-AP in mammals.1, 2
Fig.1 GPI-AP in mammals.1, 2
Biosynthesis of GPI Membrane Anchors
The first committed step in the biosynthesis of the GPI anchor occurs on the cytoplasmic face of endoplasmic reticulum (ER) membranes through a complex monoglycosyl transferase comprising seven proteins (including PIG-A). Then, after deacetylation, the obtained glucosamine-phosphatidylinositol is translocated to the luminal face and sequentially processed by ten or so proteins, to yield the mature precursor that serves for protein modification. This mature precursor possesses two side-branch EtNP attached to Man2 and Man3. At this point, the glycolipid backbone is attached to the protein C-terminus by a transamidation reaction catalyzed by a multi-subunit protein complex involving five proteins (including PIG-K). PIG-K is a caspase-like protease that cleaves proteins anchored in the ER membrane through their C-terminus while possessing a GPI attachment signal. The generated C-terminal peptide directs attachment of GPI to the ω site amino acid by transamidation involving the protein GPAA1. Importantly, during their biosynthesis, GPI-APs are also adjusted on their lipidic part in such a way that the diacyl form of phosphatidylinositol-containing sn2-linked unsaturated fatty acid is modified for a 1-alkyl-2-acyl phosphatidylinositol with an sn2- linked saturated chain.
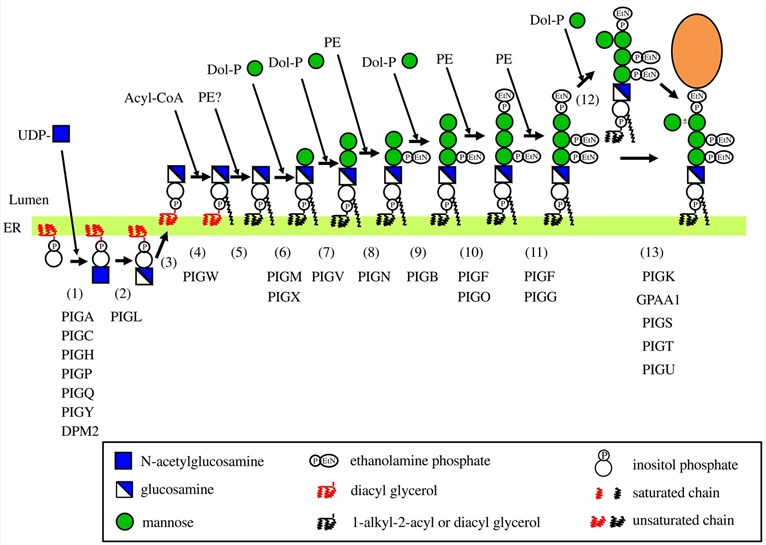 Fig.2 Biosynthesis of mammalian GPI.1, 2
Fig.2 Biosynthesis of mammalian GPI.1, 2
Interestingly, some genes involved in GPI-AP synthesis were found to be implicated in various cancers. Genes encoding for PIG-U, PIG-T, PIG-S, and GPAA1 proteins of the GPI-transamidase complex are overexpressed in almost all cancers, yet not all at the same time in specific cancer. This suggests that these genes are oncogenes and potential tumor biomarkers. Moreover, this could explain the overexpression and involvement of various GPI-APs in a diverse range of cancers.
Services at Creative Biolabs
Creative Biolabs has accumulated extensive experience from years of focusing on glycoprotein researches. We have a comprehensive technology platform and provide various glycoprotein-based services including but not limited to:
If you are focusing on glycoprotein research or you have any other questions about our services, please feel free to contact us for more information.
References
-
Kinoshita, Taroh. "Biosynthesis and biology of mammalian GPI-anchored proteins." Open biology 10.3 (2020): 190290.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 GPI-AP in mammals.1, 2
Fig.1 GPI-AP in mammals.1, 2
 Fig.2 Biosynthesis of mammalian GPI.1, 2
Fig.2 Biosynthesis of mammalian GPI.1, 2

