Biosynthesis of O-linked Glycoproteins
Overview of O-linked Glycoproteins
The glycosylation process is the covalent attachment of oligosaccharide chains on the protein backbone and is considered the most common post-translational modification of proteins. O-glycosylation corresponds to the attachment of an oligosaccharide chain to the oxygen of hydroxylated amino acid, more commonly Ser or Thr. Each glycosylated site may contain many different glycan structures. O-glycosylation in mammals represents a very diverse group of modifications that are often classified on the basis of the innermost monosaccharide. The O-glycosylation process produces an immense multiplicity of chemical structures. Each monosaccharide has 3 or 4 attachment sites for linkage of other glycan residues and can form a glycosidic linkage in an α or β configuration, allowing glycan structures to form branches. These glycan chains play key functions in biological processes, and the challenge of glycobiologists is to establish the structure/function relationship for each glycan chain.
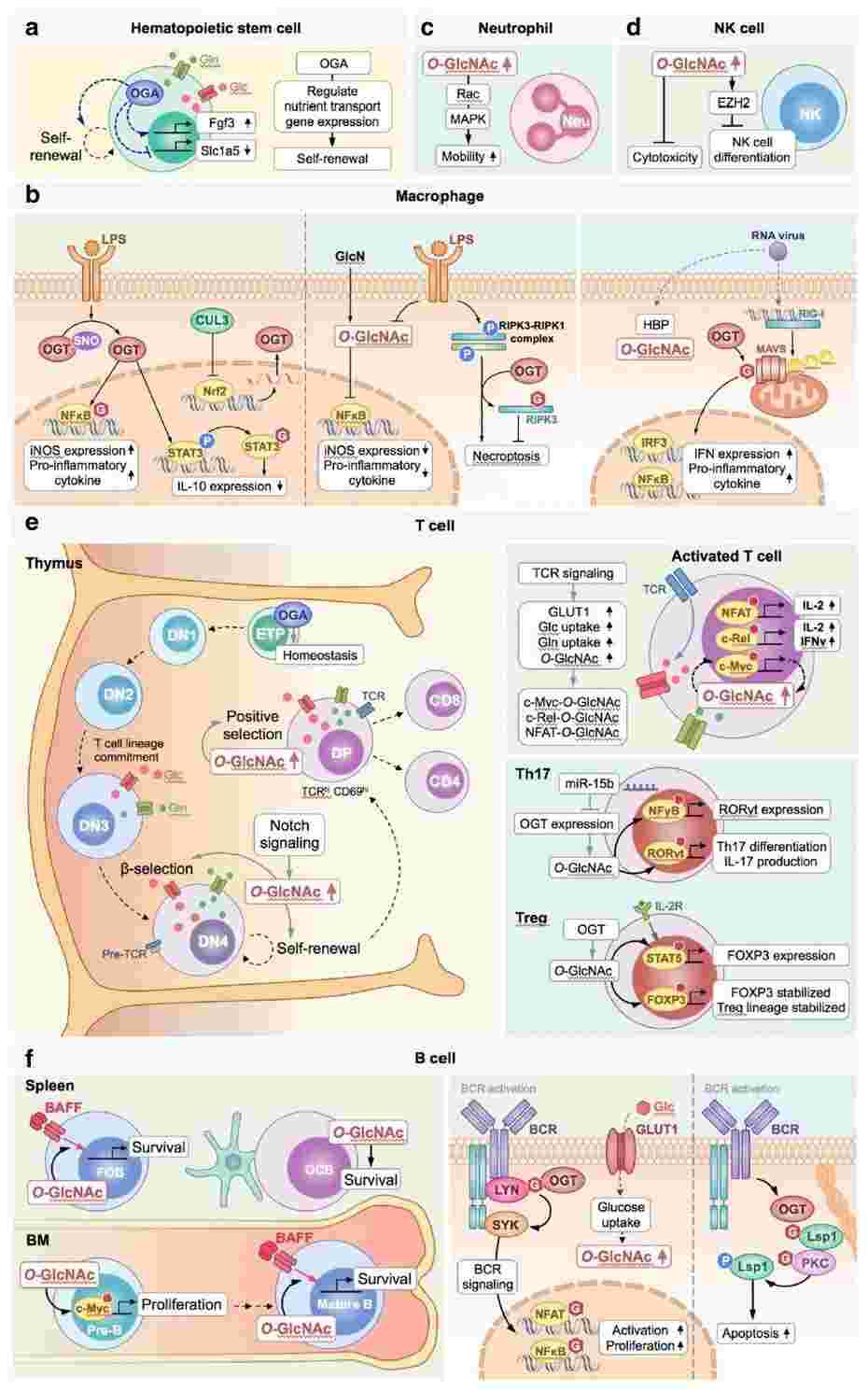 Fig.1 Role of O-GlcNAcylation in immunomodulation.1, 3
Fig.1 Role of O-GlcNAcylation in immunomodulation.1, 3
Biosynthesis of O-linked Glycoproteins
The main pathway for the biosynthesis of complex N- and O-linked glycans are located in the endoplasmic reticulum (ER) and Golgi compartments, the so-called secretory pathway. Glycosylation is restricted mainly to proteins that are synthesized and sorted in this secretory pathway, which includes ER, Golgi, lysosomal, plasma membrane, and secretory proteins. There is one exception-nuclear and cytosolic proteins can be modified with a single O-linked GlcNAc. Proteins synthesized by ribosomes and sorted in the secretory pathway are directed to the rough ER by an ER signal sequence in the NH2 terminus. After protein folding is completed in the ER, these proteins move via transport vesicles to the Golgi complex. The biosynthesis of O-glycans is initiated after the folding and oligomerization of proteins either in the late ER or in one of the Golgi compartments. Intriguingly, for the biosynthesis of glycans, no template is involved; whereas DNA forms the template for the sequence of amino acids in a protein, there is no such equivalent for the design of glycans. The biosynthesis of glycans can be divided into 3 stages. In the first stage, nucleotide glycans are synthesized in the cytoplasm. In the second stage, these nucleotide glycans are transported into the ER or the Golgi. In the third stage, specific glycosyltransferases attach the glycan to a protein or a glycan in the ER or Golgi.
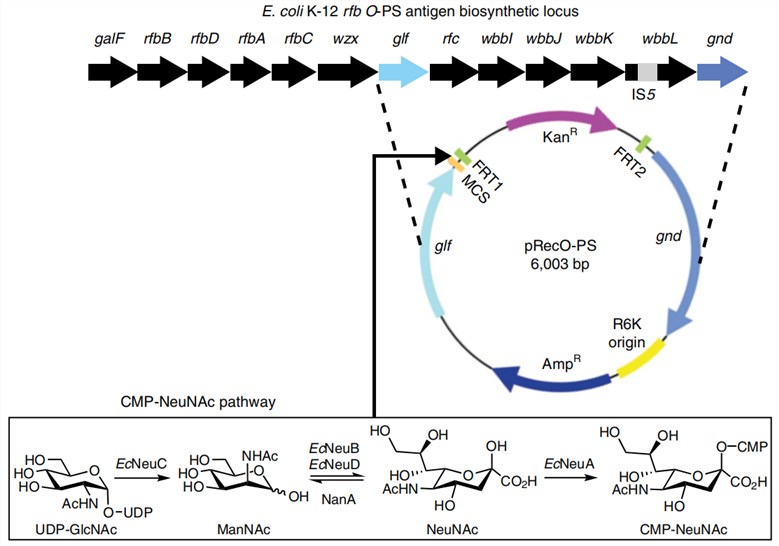 Fig. 2 Schematic representation of the biosynthesis of sialylated O-glycans.2, 3
Fig. 2 Schematic representation of the biosynthesis of sialylated O-glycans.2, 3
Services at Creative Biolabs
Focusing on glycoprotein research over years, Creative Biolabs has accumulated extensive experience from practice and developed a comprehensive technology platform. We are confident in providing every customer with satisfying services including but not limited to:
If you are focusing on glycan research and looking forward to a great partner, please don't hesitate to contact us for more information.
References
-
Chang, Yi-Hsuan, Chia-Lin Weng, and Kuo-I. Lin. "O-GlcNAcylation and its role in the immune system." Journal of biomedical science 27.1 (2020): 57.
-
Kinoshita, Taroh. "Biosynthesis and biology of mammalian GPI-anchored proteins." Open biology 10.3 (2020): 190290.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Role of O-GlcNAcylation in immunomodulation.1, 3
Fig.1 Role of O-GlcNAcylation in immunomodulation.1, 3
 Fig. 2 Schematic representation of the biosynthesis of sialylated O-glycans.2, 3
Fig. 2 Schematic representation of the biosynthesis of sialylated O-glycans.2, 3

