Biosynthesis of N-linked Glycoproteins
Overview of N-linked Glycoproteins
The N-linked glycosylation, typified by the attachment of the glycan to an asparagine side chain of the protein, is by far the most common. N-linked protein glycosylation of proteins occurs in all three domains of life. The pathway at the periplasmic membrane of prokaryotes and the process at the endoplasmic reticulum (ER) membrane is considered to be homologous. The high conservation of the process across all domains of life makes it possible to identify the underlying principles of the pathway and to develop general concepts of N-linked protein glycosylation. The consensus sequence for N-glycosylation is Asn-Xaa-Ser/Thr, where Xaa is any amino acid other than proline. The asparagine is linked to N-acetylglucosamine (GlcNAc) residues. Additional sugar residues in the glycan depend on whether the glycosylation is a high-mannose hybrid or complex type. Glycosylation imparts many properties to proteins and might be important for their function. One of the initial functions of glycosylation in a given protein is to direct the protein to the appropriate cellular location, for example, many lysosomal proteins harbor a mannose-6-phosphate moiety that is a signaling molecule. In addition, the calnexin calreticulin cycle in the ER, a quality-control mechanism that prevents misfolded proteins from being transported further, is dependent upon the N-glycosylation of the proteins in question.
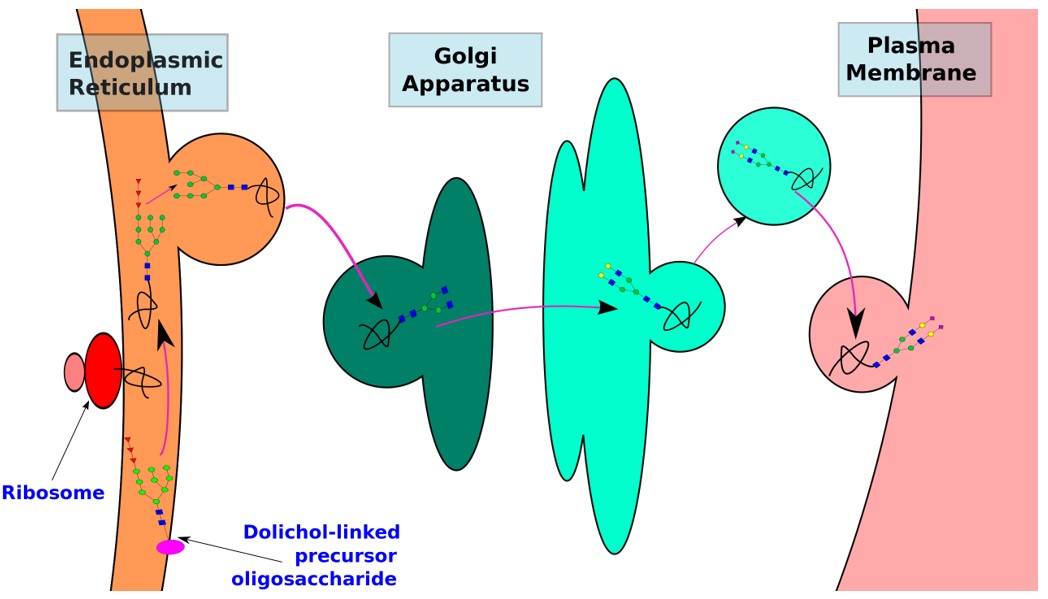 Fig.1 Biosynthetic pathways of N-linked glycoproteins.1
Fig.1 Biosynthetic pathways of N-linked glycoproteins.1
Biosynthesis of N-linked Glycoproteins
N-Glycan biosynthesis occurs in the ER and the Golgi. In the ER, an oligosaccharide assembled on Dol-P is transferred to Asn in selected Asn-X-Ser/Thr sequons of secretory and membrane proteins during their translocation into the ER. The second phase in Golgi begins with the processing of N-glycans by glycosidases and glycosyltransferases in the lumen of the ER and continues in the Golgi. In common with mammalian cells, N-glycosylation of newly synthesized proteins in insect, yeast, and plant cells starts in the ER with the transfer of the oligosaccharide precursor Glc3Man9GlcNAc2 onto selected asparagine residues that are part of the Asn-XaaSer/Thr sequon present in the growing polypeptide chain. The ensuing processing of N-glycans occurs along the secretory pathway as the glycoprotein moves from the ER through the Golgi apparatus to its final destination. The non-mammalian host cells share a few initial steps of N-glycan processing with mammalian systems, which results in the conversion of protein-linked Glc3Man9GlcNAc2 to Man8GlcNAc2. However, the resulting Man8GlcNAc2 N-glycan is further processed differently in insect, yeast, and plant cells. Processing in these nonmammalian hosts can produce N-glycans that are potentially immunogenic to humans. Although most N-glycans observed adhere to general patterns, the availability of genomic sequences has made it possible to explore a wider range of processing enzymes in insects, yeasts, and plants. Defining the functional roles of these oligosaccharide processing pathways promises to be a rich area of future endeavor in the field of glycobiology.
Services at Creative Biolabs
With rich experience and strong foundations, Creative Biolabs is capable of providing our customers with high-quality glycoprotein-based services. We have an advanced technology platform and offer comprehensive services including but not limited to:
If you are interested in our services or you have any other questions, please don't hesitate to contact us for more information.
Reference
-
Image retrieved from https://commons.wikimedia.org/wiki/File:Biosynthesis_of_N-glycan.svg, Dna, 2014, used under CC BY-SA 3.0, without any modification.
For Research Use Only.
Resources

 Fig.1 Biosynthetic pathways of N-linked glycoproteins.1
Fig.1 Biosynthetic pathways of N-linked glycoproteins.1

