Glycopeptide Ligation
Glycoproteins and glycopeptides have many biological properties, but their chemical synthesis has always been a technical challenge. Creative Biolabs has successfully established a variety of methods for manufacturing glycopeptides and has made many great achievements in this rapidly developing field. We are equipped to provide customers with a variety of effective glycopeptide synthesis services.
The synthesis of long peptides is usually accompanied by low coupling efficiency, by-products and epimer formation. These shortcomings have a serious impact on the yield and purity of the polypeptide. To overcome these limitations, a number of strategies for ligating short peptide fragments were exploited. Among the various methods, the most notable are the methods based on natural chemical ligation (NCL).
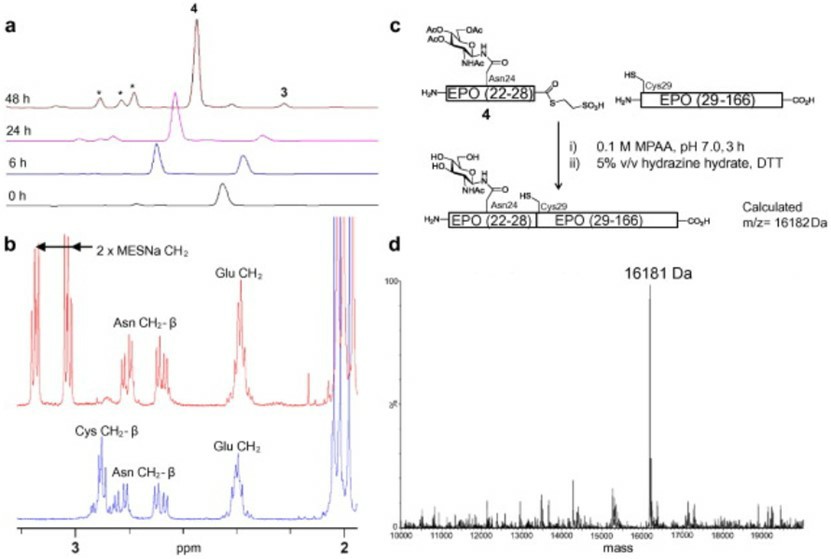 Fig.1 Native chemical ligation and analysis of N-glycopeptide thioesters.1
Fig.1 Native chemical ligation and analysis of N-glycopeptide thioesters.1
Sugar-Assisted Ligation
Sugar-assisted ligation (SAL) is proposed based on the incorporation of auxiliary thiol groups, which is compatible with synthetic glycopeptides. The auxiliary thiol group is anchored at the C-2 position of the glycopeptide. The auxiliary additional glycopeptide can mediate the ligation reaction within a wider range of amino acids at the ligation site under aqueous buffer conditions. With the advancement of technology, the second generation of SAL was developed. In the second generation of SAL, an auxiliary group is introduced at the C-3 position by forming an ester bond with thioglycolic acid. This auxiliary group can be removed by gentle hydrazinolysis after attachment so that the thiol side chain of the unprotected Cys residue remains intact. More importantly, the second-generation SAL improves the overall connection yield.
Dual Native Chemical Ligation
A novel strategy based on SAL, the Double Natural Chemical Ligation (dNCL), has gradually attracted attention, which can bypass the requirement for auxiliary additional glycosyl amino acids. In this method, the NCL reaction is used to generate auxiliary additional N-linked glycopeptide intermediates, and the resulting glycopeptide intermediates can proceed to another NCL to extend the peptide sequence. This method has been successfully applied to the synthesis of small fragments of comparable cytotoxic T lymphocyte-related antigens, and it has the potential to be used to synthesize natural glycopeptides containing complex glycan groups.
Other Glycopeptide Ligation Strategies
No matter which modified natural chemical ligation method is used, obtaining α-thioester glycopeptides is essential for the total synthesis of glycoproteins. Currently, a widely used strategy for the synthesis of α-thioester glycopeptides has been exploited. This method involves mounting the target linker on the resin before the glycopeptide extension. Furthermore, selenoester ligation is also widely used as a new alternative method in glycopeptide ligation.
Glycoproteins play a vital role in biological systems. However, glycoproteins with different properties require specific connection technologies. Creative Biolabs has developed many new technologies for the assembly of glycopeptides and glycoproteins based on traditional natural chemical connection methods. These strategies include but are not limited to SAL and dNCL. In addition, we have successfully developed a connection toolbox that can quickly and efficiently synthesize more homogeneous glycopeptides and glycoproteins. Please contact us for more details.
Reference
-
Premdjee, Bhavesh, Anna L. Adams, and Derek Macmillan. "Native N-glycopeptide thioester synthesis through N→ S acyl transfer." Bioorganic & medicinal chemistry letters 21.17 (2011): 4973-4975. Distributed under Open Access license CC BY 3.0, without modification.
For Research Use Only.
Resources

 Fig.1 Native chemical ligation and analysis of N-glycopeptide thioesters.1
Fig.1 Native chemical ligation and analysis of N-glycopeptide thioesters.1

