The distinctive structure of fucosylated oligosaccharides like 2′-fucosyllactose (2′-FL) and 3-fucosyllactose (3-FL) is propelling them to the forefront of scientific and clinical research because of their impressive health benefits. Human milk oligosaccharides (HMOs) contain vital components that support infant gut microbiota development as well as immune system modulation and defense against pathogens. Our team at Creative Biolabs acknowledges the intricate nature and promising potential of these oligosaccharides. Our extensive experience in glycoengineering and carbohydrate synthesis enables us to deliver specialized oligosaccharide-related services, including custom oligosaccharide synthesis services, oligosaccharides analysis service and tailored glycoengineering solutions, to accelerate research, product development, and clinical translation in this promising area.
2′-FL and 3-FL Oligosaccharides Structures
Fucosylated oligosaccharides represent a unique subclass of HMOs with structural attributes that define their biological specificity.
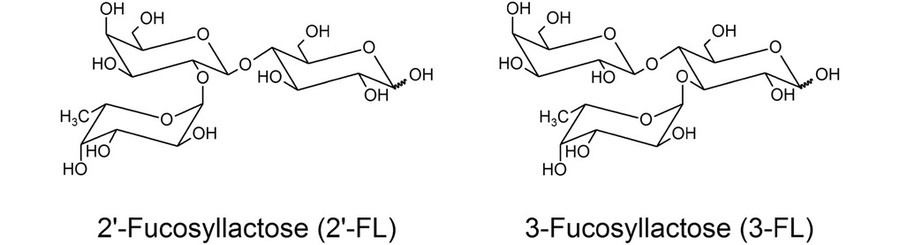 Fig.1 Structure of 2'-FL and 3-FL.1
Fig.1 Structure of 2'-FL and 3-FL.1
2'-Fucosyllactose (2′-FL)
2′-FL is a non-reducing trisaccharide composed of L-fucose, D-galactose, and D-glucose. Its structure is defined by an α-1,2 glycosidic bond between L-fucose and D-galactose (Fucα1–2Galβ1–4Glc), distinguishing it from lactose and other HMOs. Among more than 200 identified HMOs, 2′-FL is the most abundant in secretor-positive mothers, accounting for up to 45% of total HMO content.
Biologically, 2′-FL resists enzymatic hydrolysis in the small intestine, thus reaching the colon where it acts as a selective prebiotic. It supports the proliferation of Bifidobacterium longum subsp. infantis and B. breve, thereby shaping a protective gut environment during early life. In contrast to neutral HMOs like lacto-N-neotetraose (LNnT), 2′-FL has a more robust ability to block pathogen adhesion and modulate immune receptors, including dendritic cells and macrophages.
3-Fucosyllactose (3-FL)
3-FL differs in the position of its fucose residue. It features an α-1,3 glycosidic bond (Fucα1–3Galβ1–4Glc), leading to a lower abundance in human milk compared to 2′-FL. While structurally similar, its biological interactions are distinct. 3-FL binds specific bacterial lectins and has shown efficacy in antagonizing enteropathogenic E. coli strains, likely through competitive inhibition.
Moreover, 3-FL modulates Toll-like receptor 4 (TLR4) signaling, suggesting potential in immunomodulatory therapies. These findings underscore the need to study fucosylated oligosaccharides as discrete bioactives rather than redundant analogs.
Biosynthesis and Metabolic Engineering of Fucosyllactose
The increasing demand for bioidentical HMOs in nutrition and health has driven innovation in microbial biosynthesis and synthetic biology platforms.
Microbial Fermentation for 2′-FL Production
The most scalable strategy for 2′-FL production involves genetically modified E. coli strains equipped with GDP-L-fucose biosynthetic genes and α-1,2-fucosyltransferase. Under optimized fed-batch fermentation, titers have reached industrially relevant levels exceeding 120 g/L, with glycerol and glucose serving as efficient carbon sources. Such production systems are approved by primary regulatory authorities. However, scale-up continues to face obstacles such as metabolic byproduct accumulation, downstream purification, and cost-efficiency. Creative Biolabs supports fucosylated oligosaccharide manufacturing through advanced microbial glycoengineering services, offering pathway optimization, strain development, and fermentation scale-up solutions for 2′-FL and beyond.
Synthetic Biology Innovations for 3-FL and Hybrid Oligosaccharides
Producing 3-FL and other hybrid fucosylated oligosaccharides involves a deeper layer of metabolic reprogramming.Gene-editing technologies and other advanced synthetic biology tools have been employed to fine-tune host strains such as Bacillus subtilis and E. coli, introducing highly specific α-1,3-fucosyltransferases and optimizing nucleotide sugar pathways. Additionally, membrane transporters and glycosylation bottlenecks are re-engineered to enhance yield and structural fidelity. Metabolic flux studies have underscored the importance of pathway balancing and precursor regeneration in achieving high-titer oligosaccharide production. Notably, yields of 3-FL up to 22.85 g/L have been reported under controlled fermentation conditions—yet industrial scalability remains a work in progress.
At Creative Biolabs, we provide a comprehensive array of synthetic biology and glycoengineering services for cell line, allowing you to precisely modify microbial, mammalian, insect, yeast, or plant systems to unlock the full biosynthetic potential of fucosylated oligosaccharides. Whether you're optimizing your chassis strain or building next-gen oligosaccharides from scratch, we've got your back with full-stack glycoengineering support.
Future Directions
Emerging research supports the broad application of fucosylated oligosaccharides in clinical nutrition, gut health, and immune modulation.
Tailoring HMO Formulations for Disease-Specific Applications
Precision nutrition is moving toward customized oligosaccharide blends, such as 2′-FL + 3-FL, tailored to address complex diseases. For example, in inflammatory bowel disease (IBD), these oligosaccharides may reduce epithelial permeability and enhance Treg differentiation. In metabolic syndrome, they modulate energy balance via gut-brain axis signaling. Combining fucosyllactose with short-chain fatty acids (SCFAs) or aryl hydrocarbon receptor (AhR) agonists may amplify anti-inflammatory effects. Biomarker-driven dosing strategies offer an individualized approach that aligns with the principles of functional nutrition.
Rare Fucosylated Oligosaccharides
While 2′-FL and 3-FL dominate research, lesser-known HMOs like difucosyllactose and lacto-N-fucopentaose deserve focused exploration. Their low natural abundance and synthesis complexity pose challenges for large-scale use but offer unique opportunities for niche therapeutic applications. Cutting-edge platforms can screen for novel fucosyltransferase activities and pathway bottlenecks, accelerating access to these rare structures. Structural characterization through tandem mass spectrometry and nuclear magnetic resonance (NMR) remains essential for functional validation. We don't just provide services—we help you design, develop, and deliver oligosaccharide solutions that work. If you need custom oligosaccharide synthesis or strain design for oligosaccharide production, check our fucosylated oligosaccharide services now!
Fucosyllactose serves as a fundamental element within the field of modern glycobiology and functional nutrition. Through the discovery of their unique α-linked structures up to the development of biosynthesis techniques and translational applications 2′-FL and 3-FL demonstrate the therapeutic power of human milk oligosaccharides. Creative Biolabs supports oligosaccharide innovation through dedicated research and development. Our comprehensive platforms deliver custom synthesis and oligosaccharide structural analysis together with glycoengineering capabilities to enable researchers, biotech startups, and nutrition companies to introduce next-generation products based on oligosaccharides to the marketplace. Our experts are available to help you advance your fucosyllactose research and product development process.
Reference
-
Christensen, Anne Støvlbæk, et al. "Quantifying the human milk oligosaccharides 2'‐fucosyllactose and 3‐fucosyllactose in different food applications by high‐performance liquid chromatography with refractive index detection." Journal of Food Science 85.2 (2020): 332-339. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1111/1750-3841.15005
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Structure of 2'-FL and 3-FL.1
Fig.1 Structure of 2'-FL and 3-FL.1

