Introduction
Protein glycosylation, specifically Fc glycosylation, is a crucial post-translational modification (PTM) that significantly impacts the structural integrity, functional efficacy, and therapeutic performance of biopharmaceuticals, particularly monoclonal antibodies (mAbs). The Fc region of immunoglobulins (IgG) plays a key role in mediating immune responses through mechanisms such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). In-depth analysis and control of Fc glycosylation are essential for ensuring the desired functional outcomes of glycosylated biologics.
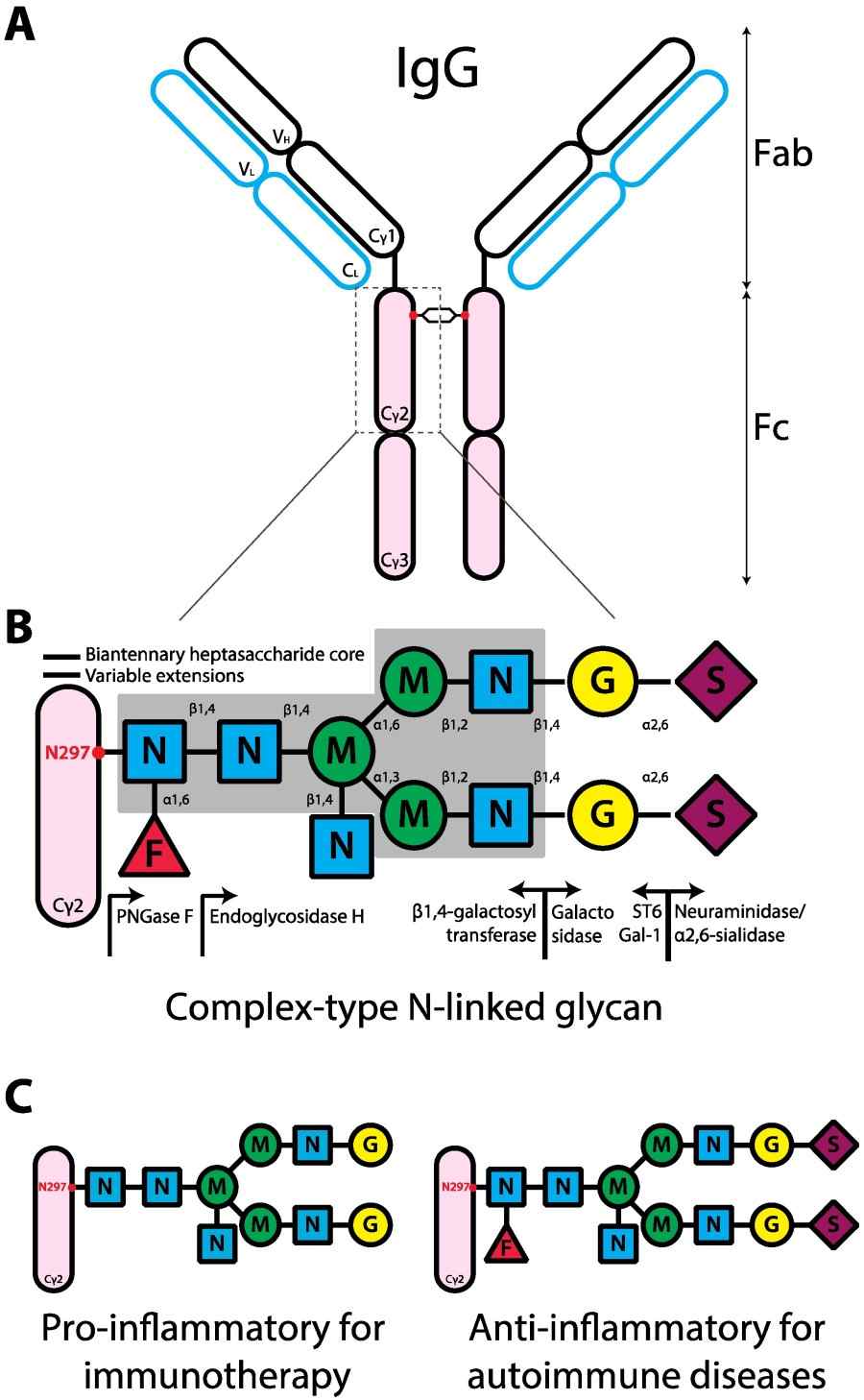 Fig.1 The human IgG structure and glycan composition.1
Fig.1 The human IgG structure and glycan composition.1
The Importance of Fc Glycosylation
Fc glycosylation refers to the attachment of glycans to the Fc region of antibodies, particularly the Asn297 residue, a conserved site in the CH2 domain of IgG. This modification not only influences the antibody's stability but also its interaction with Fcγ receptors (FcγRs) and the complement system, both of which are essential for its effector functions. Changes in the glycosylation patterns of N297 glycosylation can lead to significant differences in antibody performance, impacting both its pharmacokinetics (PK) and pharmacodynamics (PD).
Key Glycosylation Modifications
-
N-glycosylation at Asn297 (N297 glycosylation): Affects antibody binding to FcγRIIIa, influencing ADCC.
-
Core fucosylation: The addition of fucose in the glycan structure at Asn297 reduces the binding affinity to FcγRIIIa, thus dampening ADCC activity.
-
Sialylation and Galactosylation: These modifications influence the anti-inflammatory properties and half-life of the antibody in circulation.
-
Mannose Trimming: This can influence immune cell recognition and effector functions
Fc Glycosylation in Biopharmaceuticals
Therapeutic mAbs, including Herceptin (Trastuzumab), Etanercept, and other biologics, often have N297 glycosylation at the core of their functionality. These glycosylation patterns must be tightly controlled during production to ensure consistent product efficacy. For example, Herceptin glycosylation can directly influence its ability to trigger immune responses, while Etanercept glycosylation is crucial for its interaction with soluble TNF receptors.
Acceptable changes in the quality attributes of glycosylated biopharmaceuticals, such as variations in glycosylation, are critical to ensuring product consistency and regulatory compliance. A detailed and robust glycosylation analysis helps in:
-
Ensuring batch-to-batch consistency.
-
Optimizing glycan profiles to enhance therapeutic performance.
-
Minimizing immunogenicity associated with altered glycosylation.
Our Fc Glycosylation Analysis Service
At Creative Biolabs, we offer an advanced Fc glycosylation analysis service that combines cutting-edge techniques to assess the glycosylation status of biopharmaceuticals. Using mass spectrometry (MS), high-performance liquid chromatography (HPLC), and lectin-based assays, we provide comprehensive insights into the glycan structures attached to the Fc region of antibodies:
Benefits of Fc Glycosylation Analysis
-
Detailed glycosylation analysis ensures that antibodies are optimized for their intended immune functions.
-
Ensure that glycosylation profiles comply with regulatory standards for acceptable changes in quality attributes.
-
Monitor glycosylation during production to optimize yields and reduce unwanted heterogeneity.
Our Glycosylation Analysis Includes:
-
Intact glycoprotein analysis: To assess the glycosylation profile of the antibody at the whole protein level.
-
Site-specific glycopeptide mapping: Identifying the exact glycosylation sites and types of glycans attached to Asn297.
-
Glycan profiling: Characterizing the glycan composition attached to the Fc region.
-
Quantification of glycan composition: Ensuring the correct distribution of glycan structures that influence antibody effector functions.
Why Choose Us?
At Creative Biolabs, we bring over several years of experience in the biotechnology, specializing in advanced glycosylation analysis. Our Fc glycosylation analysis service is designed to provide the highest level of accuracy, reliability, and efficiency in glycosylation profiling. Here's why you should choose us:
-
We use cutting-edge techniques, including mass spectrometry (MS) and HPLC, to provide precise, high-quality glycosylation profiling.
-
Our services are tailored to meet your specific needs, whether for mAb glycosylation or other glycoprotein analysis, ensuring optimal results.
-
We ensure your products meet regulatory standards, offering data that supports safe, effective, and consistent biopharmaceutical development.
-
We deliver quick, reliable results at competitive prices, helping you move forward efficiently with your research and development.
Published Data
Fc Region Glycosylation Analysis plays a critical role in understanding the pro-inflammatory properties of Anti-Citrullinated Protein Antibodies (ACPAs) in Rheumatoid Arthritis (RA). This study investigated how the N-glycosylation pattern of the IgG Fc region in ACPAs influences their ability to induce the production of TNFα, a key inflammatory cytokine. The analysis involved digesting isolated IgG and ACPA-IgG samples with trypsin, followed by glycosylation pattern analysis using nano-UHPLC-MS/MS. The results revealed significant differences in the glycosylation patterns of ACPA-IgG Fc regions compared to healthy IgG, including lower levels of fucosylation, galactosylation, and sialylation. Furthermore, the glycosylation patterns in ACPA-IgG correlated negatively with inflammatory markers like CRP, ESR, and RF, with the G0 glycoform (lacking galactose and terminal sialic acid) showing a strong negative correlation with TNFα production. A detailed comparison of the galactosylation and sialylation levels across IgG subclasses (IgG1, IgG2, and IgG3) in RA patients and healthy controls indicated that ACPA-IgG consistently exhibited lower glycosylation levels, particularly in IgG1, highlighting the importance of Fc region glycosylation in regulating immune responses and inflammation in RA. This analysis underscores the significance of Fc glycosylation in disease pathogenesis and its potential as a biomarker for inflammatory diseases.
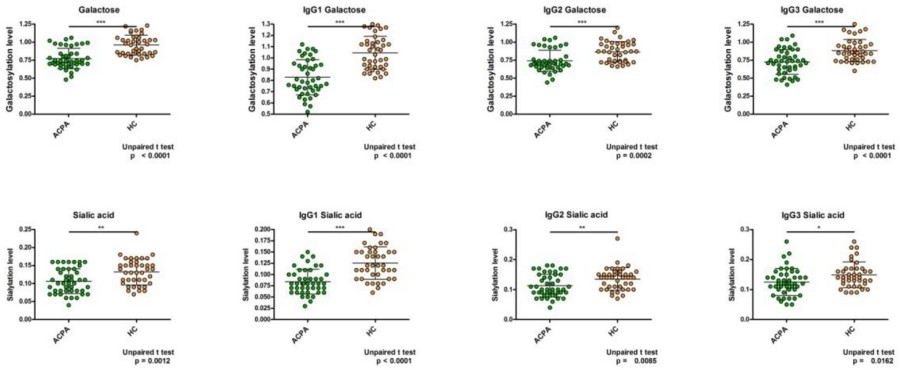 Fig.2 Comparison of galactosylation and sialylation levels in ACPA-IgG and healthy IgG subclasses.2
Fig.2 Comparison of galactosylation and sialylation levels in ACPA-IgG and healthy IgG subclasses.2
Fc glycosylation is a crucial determinant of the efficacy and safety of therapeutic antibodies. At Creative Biolabs, our comprehensive Fc glycosylation analysis service provides deep insights into the glycosylation patterns of monoclonal antibodies, and other glycoproteins. By utilizing advanced analytical techniques and methodologies, we help ensure that your glycosylated biopharmaceutical products meet the highest standards for efficacy, safety, and regulatory compliance. If you would like to learn more about our Fc glycosylation analysis service, please contact us today!
References
-
Shade, Kai-Ting C., and Robert M. Anthony. "Antibody glycosylation and inflammation." Antibodies 2.3 (2013): 392-414. Distributed under Open Access license CC BY 3.0, without modification. https://doi.org/10.3390/antib2030392
-
Gyebrovszki, Balázs, et al. "The role of IgG Fc region N-glycosylation in the pathomechanism of rheumatoid arthritis." International journal of molecular sciences 23.10 (2022): 5828. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/ijms23105828
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 The human IgG structure and glycan composition.1
Fig.1 The human IgG structure and glycan composition.1
 Fig.2 Comparison of galactosylation and sialylation levels in ACPA-IgG and healthy IgG subclasses.2
Fig.2 Comparison of galactosylation and sialylation levels in ACPA-IgG and healthy IgG subclasses.2

