Introduction of O-Glycosylation Analysis
O-glycosylation, a key post-translational modification, involves attaching glycans to serine or threonine residues in proteins. This process doesn't just tweak proteins—it shapes how they work, how stable they are, and how they interact with other molecules. It's crucial for cell signaling, immune responses, and even how diseases develop. Unlike N-glycosylation, which has a clear consensus sequence, O-glycosylation is trickier. It depends on the protein's local environment, making it more complex to study. From the mucin-type glycans that protect our epithelial cells to the O-GlcNAc modifications that regulate nuclear processes, these sugars play many roles. But to understand them, you need the right tools and know-how. At Creative Biolabs, our O-glycosylation analysis services are built to tackle this complexity head-on. Whether you're hunting for disease biomarkers, improving therapeutic proteins, or decoding how cells communicate, we've got you covered. Our services blend cutting-edge tech with years of expertise to turn your samples into insights you can use.
Types of O-Glycosylation
Mucin-Type O-Glycosylation
This is the most common type, starting with a sugar called GalNAc attaching to Ser/Thr. It's big in mucin proteins, which line our organs and protect them with a slimy layer. But when things go wrong here, diseases can follow. Abnormal mucin glycosylation shows up in cancer—think of Tn and sialyl-Tn antigens used to detect gastric or breast cancer. It also plays a role in autoimmune diseases and viral infections, like how SARS-CoV-2 uses glycan changes to infect cells.
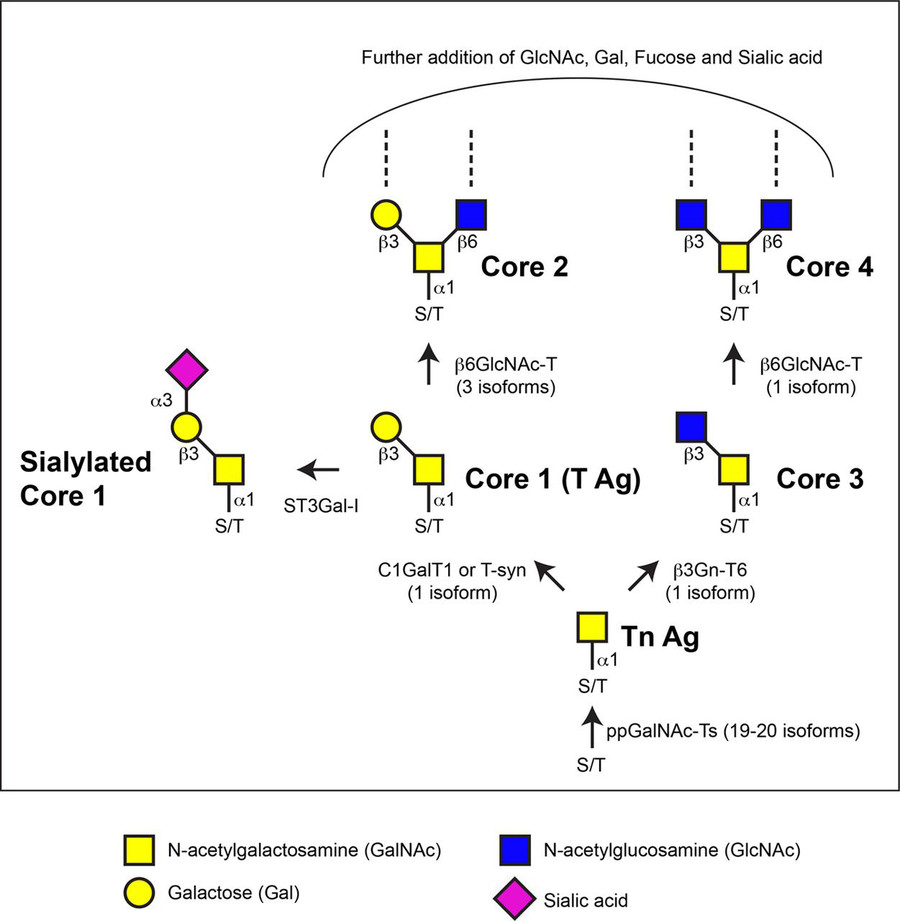 Fig.1 Biosynthesis process of mucin-type O-glycans.1
Fig.1 Biosynthesis process of mucin-type O-glycans.1
Other Important Types
-
O-linked fucosylation
: Adds fucose to proteins, influencing how immune cells move and recognize pathogens.
-
O-GlcNAcylation
: Tags nuclear and cytoplasmic proteins with GlcNAc, controlling things like gene transcription and how cells handle stress.
-
Collagen glycosylation
: Strengthens the extracellular matrix, vital for tissue health and preventing issues like fibrosis.
Figuring out which type you're dealing with needs precise methods. Our team uses techniques like lectin affinity and chemoenzymatic tagging to pull out specific glycopeptides, making sure we classify your sample's O-glycosylation correctly.
Prepping Your Samples: The First Step to Good Data
Great analysis starts with great samples. We work with all sorts of biological materials, but each needs special care to keep those glycans intact:
|
Sample Type
|
What It Is
|
How to Handle It
|
|
Cell Lysates
|
Cells or tissues broken down into fluid
|
Use gentle buffers with protease inhibitors—no harsh stuff!
|
|
Plasma/Serum
|
Blood fluids with circulating proteins
|
Freeze at -80°C and use small aliquots to avoid thawing and refreezing.
|
|
Tissue Extracts
|
Homogenized tissues (like liver or tumors)
|
Use methods that fit your tissue type to get those glycoproteins out safely.
|
|
Biofluids
|
Urine, CSF, saliva—even spit!
|
If glycoproteins are rare, concentrate them first.
|
|
Recombinant Proteins
|
Lab-engineered proteins
|
Tell us how they were made (expression system) so we can analyze them in context.
|
Pro Tips:
-
For tissues/cells: Soft lysis buffers are your friend—they protect glycans from breaking down.
-
If N-glycans are messing things up: Use PNGase F to remove them first. It makes isolating O-glycopeptides a breeze.
Not sure which sample to send? Don't stress—our experts are here to help you pick the right one and tell you details about the proper preservation methods that ensure your sample activity.
Our Analysis Workflow: From Sample to Insight
1. Enriching Glycopeptides
O-glycopeptides are often rare in samples, so we need to pull them out efficiently:
-
Hydrazide Chemistry: Grabs glycopeptides by their reducing ends—great for big-scale studies.
-
Lectin Affinity: Uses sugar-binding proteins (lectins) to catch specific glycans, like mucin-type ones.
-
Chemoenzymatic Tagging: Labels O-glycans with enzymes for sensitive detection, perfect for small samples.
2. Quantifying with Isotope Tags
For different samples, we use:
-
SILAC: Labels cells with heavy isotopes to see how glycan levels change under different conditions.
-
TMT/iTRAQ: Tags up to 10 samples at once for high-throughput analysis—no time wasted!
3. Unlocking Structures
-
Mass Spectrometry (MS): Our high-res Orbitrap and Q-TOF machines decode glycopeptide fragments to find exactly where and what the glycans are.
-
NMR Spectroscopy: Shows the 3D shape and movement of glycans, key for understanding how they bind to proteins.
-
Lectin Microarrays: Tests many glycans at once to find binding patterns—super useful for discovering biomarkers.
We don't just follow a checklist. We work with you to design experiments that answer your questions, ensuring every step is optimized for your goals.
Cutting-Edge Tools
-
High-Resolution Cellular Glycomics: See O-glycosylation differences at high cellular resolution—ideal for rare cells or tracking cell changes.
-
Gene-editing Glycoengineering: Tweak cell glycosylation pathways to make proteins with the glycans you want, speeding up therapeutic development.
-
AI-Driven Prediction: Use advanced tools and our own algorithms to predict O-glycosylation sites before experiments, saving time and guesswork.
Advantages of Our Services
-
End-to-End Service: We handle your entire sample process internally and maintain full control without the need for external partners.
-
Top-Notch Tech: The latest MS technology and NMR and lectin arrays combined with our proprietary methods deliver superior sensitivity.
-
Customized for You: We customize our methods to match your project requirements whether you require basic site mapping or detailed structural analysis.
-
Expert Team: Our glycomics experts provide support throughout each phase from planning to results interpretation.
O-glycosylation might be complex, but do not worry. At Creative Biolabs, we're here to make your research easier and more impactful. Whether you're in basic science or translating findings to the clinic, our services are designed to help you unlock the secrets of these sugar modifications. Let's turn those tricky O-glycosylation questions into clear answers—together, we'll make sure your analysis is as powerful as your science. Contact us today to start your O-Glycosylation Analysis journey!
Reference
-
Tran, Duy T., and Kelly G. Ten Hagen. "Mucin-type O-glycosylation during development." Journal of Biological Chemistry 288.10 (2013): 6921-6929. Distributed under Open Access license CC BY 4.0, without modification. https://www.sci-hub.ru/10.1074/jbc.R112.418558
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Biosynthesis process of mucin-type O-glycans.1
Fig.1 Biosynthesis process of mucin-type O-glycans.1

