Introduction: Glycosylation as a Molecular Signature of Aging
Glycosylation, a key post-translational modification, is the enzymatic process of adding glycans to proteins and lipids. In contrast, glycation is a natural process characterized by non-enzymatic glycation that produces advanced glycation end products (AGEs), which accumulate as people age. These two types of glycosylation changes form the biochemical foundation of the "glycosylation theory of aging", which connects structural carbohydrate changes to age-related diseases, inflammation, and metabolic issues. As a CRO focused on glycoscience, Creative Biolabs offers precise glycosylation profiling solutions to support your aging-related research.
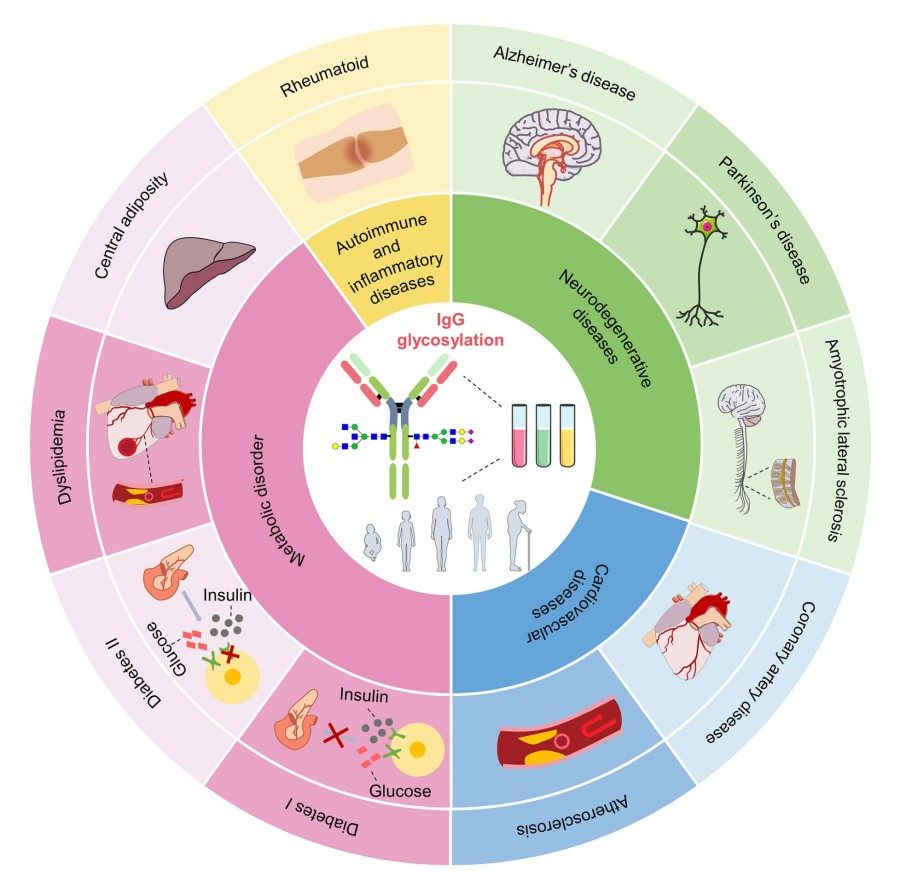 Fig.1 IgG glycosylation in aging and disease.1
Fig.1 IgG glycosylation in aging and disease.1
The Glycosylation–Inflammation Feedback Loop
Aging is not merely the passage of time—it's an entropic process often fueled by inflammation. Glycosylation serves as a molecular link between immune homeostasis and dysregulation:
-
Enzymatic glycosylation shifts increase inflammatory potential
-
Non-enzymatic glycation (AGEs) directly promotes cytokine cascades via the Receptor for AGE (RAGE)
-
Both mechanisms amplify immunosenescence, reduce pathogen clearance, and impair repair mechanisms
Emerging data suggest that inflammation alters glycosylation enzyme expression, creating a self-reinforcing loop that accelerates biological aging and disease progression. This is particularly relevant in:
-
Rheumatoid arthritis: IgG-G0 levels correlate with disease activity
-
Atherosclerosis: AGE–RAGE signaling promotes vascular inflammation
-
Neurodegeneration: AGEs accumulate in Alzheimer's disease plaques
Metabolic and Tissue-Level Implications
Altered glycosylation reflects deeper metabolic disruptions. Hyperglycemia and oxidative stress accelerate glycation reactions, particularly in diabetes mellitus. Basement membrane glycation in the kidney impairs filtration, contributing to diabetic nephropathy. In the retina, AGEs disrupt pericyte-endothelial interactions, leading to microaneurysms. Moreover, AGE-modified proteins are resistant to proteolysis, leading to intracellular accumulation and lysosomal dysfunction—hallmarks of aging. Crosslinked AGEs stiffen vasculature and skin, limiting elasticity and promoting mechanical stress injuries. Understanding these mechanisms opens avenues for therapeutic intervention using AGE inhibitors, RAGE antagonists, and glycosylation modulators.
Enzymatic Glycosylation Changes in Aging: The IgG-G0 Formation
Among the most well-studied examples of age-related glycosylation is N-glycan remodeling on IgG. With increasing age, human IgG molecules show a higher proportion of agalactosylated N-glycans (termed IgG-G0), which lack terminal galactose residues. These structures are more than passive markers; they actively modulate immune functions. IgG-G0 is associated with:
-
Pro-inflammatory Fc receptor binding, enhancing immune cell activation
-
Complement pathway activation via mannose-binding lectin
-
Increased affinity for dendritic lectin receptors, amplifying cytokine signaling
These IgG variants lack galactose and sialic acid residues and often carry bisecting GlcNAc without core fucose (left side of Fig.2), shifting the immune balance toward a pro-inflammatory phenotype. In contrast, glycoforms with core fucosylation, galactosylation, or terminal sialylation (right side of Fig.2) promote anti-inflammatory signaling.
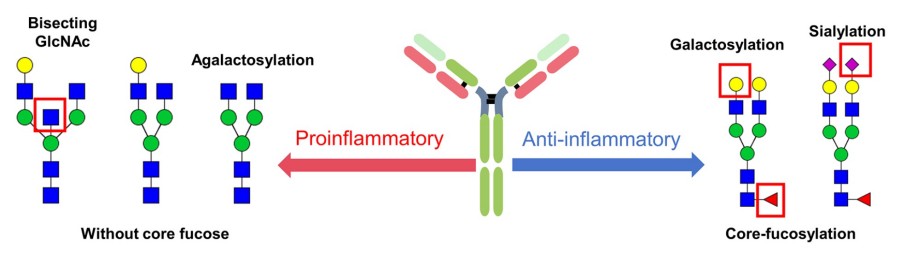 Fig.2 IgG glycosylation and inflammatory responses.1
Fig.2 IgG glycosylation and inflammatory responses.1
This shift promotes a chronic low-grade inflammatory state known as inflammaging, a hallmark of biological aging that correlates with frailty, cardiovascular risk, and impaired vaccine responses. Creative Biolabs offers high-resolution Fc glycosylation profiling, enabling detection of IgG glycoform shifts across aging cohorts, autoimmune diseases, or therapeutic monoclonal antibodies.
Non-Enzymatic Glycosylation and AGE Accumulation
In contrast to enzymatic glycosylation, non-enzymatic glycation—the covalent attachment of reducing sugars to proteins—leads to the formation of AGEs. This irreversible process is especially prevalent in long-lived structural proteins such as collagen and laminin, notably in:
-
Basement membranes of kidneys, nerves, and vasculature
-
Lens crystallin, contributing to cataractogenesis
-
Myocardial and arterial ECM, promoting stiffness and hypertension
AGEs exert harmful effects by crosslinking extracellular matrix components and activating RAGE on immune and endothelial cells, triggering:
-
NF-κB–dependent pro-inflammatory transcription
-
Oxidative stress and ROS generation
-
Vascular dysfunction and tissue remodeling
The accumulation of AGEs with age and in diabetes illustrates a damaging feed-forward cycle where hyperglycemia and inflammation accelerate tissue degeneration. Creative Biolabs provides quantitative AGE detection and non-enzymatic glycation assays, supporting studies in diabetic aging, nephropathy, and vascular biology.
Analytical Techniques for Glycosylation Research
With advanced glycomics technologies, it is now possible to quantitatively track glycosylation changes over time and correlate them with clinical phenotypes. Creative Biolabs integrates mass spectrometry, lectin profiling, and glycoarray technologies, enabling comprehensive glycosylation characterization for aging-related translational studies.
|
Glycosylation Marker
|
Role in Aging/Inflammation
|
Analytical Platform
|
|
IgG-G0
|
Inflammaging biomarker
|
HILIC-HPLC, MS
|
|
Total AGE levels
|
Diabetic & renal aging risk
|
ELISA, LC-MS
|
|
Sialylation
|
Immunomodulation capacity
|
Lectin arrays, MALDI
|
|
Galectin-binding glycans
|
Senescence signaling
|
SPR, glycan microarray
|
Glycosylation—both enzymatic and non-enzymatic—provides a molecular window into the aging process. By influencing immune tone, metabolic resilience, and tissue architecture, glycan structures regulate how we age and how age-related diseases manifest. At Creative Biolabs, we are dedicated to advancing aging research through state-of-the-art glycomics platforms, customized analytical services, and expert scientific consultation. Whether you are investigating IgG glycoforms, AGE accumulation, or glycan-mediated inflammation, we deliver the precision you need to decode the glycobiology of aging.
Reference
-
Wu, Yongqi, et al. "Immunoglobulin G glycosylation and its alterations in aging-related diseases: IgG glycosylation with aging-related diseases." Acta Biochimica et Biophysica Sinica 56.8 (2024): 1221. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3724/abbs.2024137
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 IgG glycosylation in aging and disease.1
Fig.1 IgG glycosylation in aging and disease.1
 Fig.2 IgG glycosylation and inflammatory responses.1
Fig.2 IgG glycosylation and inflammatory responses.1

