Carbohydrate-related protein modifications play divergent roles in biology. Enzymatic glycosylation, a controlled and site-specific process, is vital for correct protein folding, structural integrity, and biological activity. In contrast, non-enzymatic glycosylation, also known as glycation, occurs spontaneously and can impair protein function through the irreversible formation of advanced glycation end products (AGEs). Differentiating between these two processes is not a minor technicality—it's essential for research in biologics manufacturing, diabetes, aging, neurodegeneration, and cancer. At Creative Biolabs, we offer a full suite of analytical tools to help researchers precisely characterize both enzymatic glycosylation and pathological glycation events, ensuring reliable data for mechanistic insight and product quality assessment.
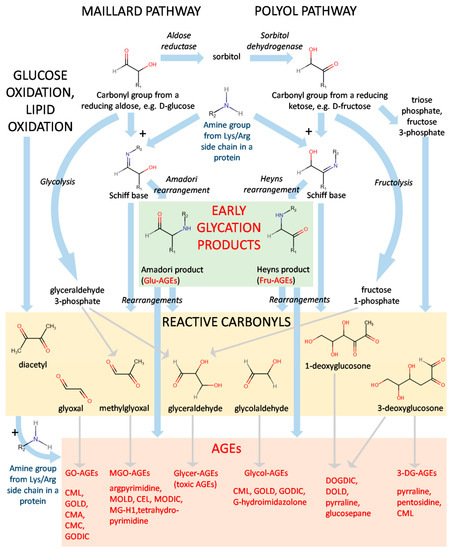 Fig.1 AGE formation via glycation.1,3
Fig.1 AGE formation via glycation.1,3
What Is Non-Enzymatic Glycosylation (Glycation)?
Glycation begins when reducing sugars such as glucose react non-enzymatically with free amino groups—typically on lysine or arginine residues—on proteins. This forms Schiff bases, which quickly rearrange into Amadori products. Over time, these early adducts evolve through oxidation and molecular crosslinking into AGEs. These AGEs often accumulate on long-lived proteins like collagen, hemoglobin, and crystallin. Unlike enzymatic glycosylation, glycation is uncontrolled, irreversible, and often accelerated under hyperglycemic or oxidative stress. The biological impact is significant, potentially leading to:
-
Altered enzymatic function
-
Increased protein aggregation
-
Activation of immune signaling through RAGE (Receptor for Advanced Glycation End products) and related receptors
At Creative Biolabs, we help clients trace these modifications at molecular resolution using our advanced analytical workflows.
What Are Advanced Glycation End Products (AGEs)?
AGEs are chemically diverse end-products formed through prolonged glycation and oxidative stress. They are typically found in aged tissues, diabetic patients, and heat-processed foods. AGEs are not just structural anomalies—they actively participate in pathophysiology by interacting with specific receptors, notably RAGE, activating pro-inflammatory and pro-fibrotic pathways.
Key Pathological Roles of AGEs
-
Diabetes: AGE accumulation in vascular basement membranes contributes to microvascular complications.
-
Aging: Crosslinking of ECM proteins reduces tissue elasticity.
-
Neurodegeneration: AGEs co-localize with amyloid plaques and enhance tau phosphorylation.
-
Cancer: RAGE signaling can promote tumor-associated inflammation and metastasis.
Representative AGEs and Their Detection Significance
Understanding and detecting specific AGE types is crucial for both clinical research and quality control in protein-based products. Common AGE structures include:
|
AGE Name
|
Precursor
|
Biological Relevance
|
|
CML (Carboxymethyllysine)
|
Glyoxal, Amadori products
|
Inflammatory marker, renal disease biomarker
|
|
Pentosidine
|
Pentose sugars
|
Protein crosslinking, aging indicator
|
|
Glucosepane
|
Glucose, arginine/lysine
|
Major collagen crosslink in aged tissues
|
|
MG-H1 (Methylglyoxal-Hydroimidazolone)
|
Methylglyoxal
|
Diabetic tissue stress marker
|
Why AGEs Detection Matters?
-
CML and MG-H1 are frequently used as biomarkers for oxidative and glycemic stress.
-
Pentosidine serves as a cumulative indicator of chronic AGE exposure.
-
Glucosepane plays a role in irreversible matrix stiffening and is a target for anti-aging therapeutics.
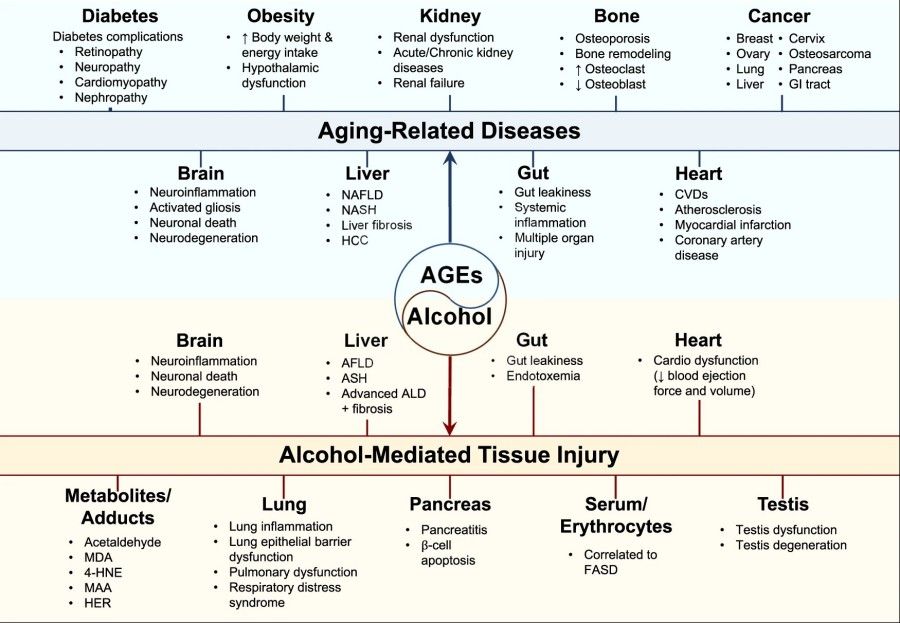 Fig.2 AGE accumulation in aging and alcohol-induced organ damage.2,3
Fig.2 AGE accumulation in aging and alcohol-induced organ damage.2,3
Analytical Expertise Supporting AGE Research
To assess glycation and AGE formation, high-resolution detection is essential. At Creative Biolabs, we integrate multiple orthogonal platforms for unambiguous identification and quantification of glycated residues and AGE-related crosslinks. Together, these platforms offer a robust solution for exploring glycation pathways, characterizing AGE burden, and supporting the development of glycation-targeted interventions.
-
Mass Spectrometry (MS): Our MS-based services enable site-specific identification of glycation sites and AGE-adduct profiling, with high mass accuracy and sensitivity. From intact protein MS to peptide-level mapping, we provide tailored glycation characterization protocols for both native and recombinant proteins.
-
Liquid Chromatography-Electrospray Ionization-MS (LC-ESI-MS): Ideal for quantitative profiling of AGE intermediates and advanced products, LC-ESI-MS offers powerful separation coupled with soft ionization, enabling detailed glycation fingerprinting even in complex biological matrices.
-
High-Performance Liquid Chromatography (HPLC): Our HPLC platforms support AGE detection via fluorescence or UV detectors, offering excellent resolution for AGEs like CML, pentosidine, and MG-H1. We customize chromatographic conditions based on protein type and modification extent.
-
Fourier Transform Infrared (FTIR) Spectroscopy: For clients seeking label-free, non-destructive AGE analysis, FTIR spectroscopy provides rapid detection of AGE-specific carbonyl stretches and crosslinking signals in protein materials.
-
Lectin Microarray: While not directly targeting AGEs, this platform enables glycan-binding pattern profiling, helping differentiate native glycosylation loss from AGE-associated structural alterations. It's especially useful in functional glycoprotein screening under stress conditions.
Creative Biolabs' Expertise in Glycosylation and Glycation Profiling
At Creative Biolabs, we offer integrated solutions to differentiate and characterize glycation and glycosylation events, especially in biopharmaceutical samples.
-
N-/O-glycosylation mapping
-
Glycopeptide profiling
-
Sialic acid quantification
-
Glycosylation-related stability studies
Custom AGE Analysis
-
Site-specific glycation quantification
-
AGE accumulation profiling in recombinant proteins or tissues
-
Tailored AGE-RAGE signaling assays
Why Partner with Creative Biolabs?
Creative Biolabs stands apart in the glycoscience CRO space. With over two decades of experience in protein glycosylation and post-translational modification analysis, we offer:
-
Deep expertise in glycation-related biomarker discovery
-
Customizable protocols tailored to your protein or disease model
-
Integrated platforms for both qualitative profiling and quantitative validation
-
Fast turnaround, reliable interpretation, and regulatory-grade reporting
Whether you're investigating AGE accumulation in metabolic disease, evaluating glycation stress in protein therapeutics, or mapping protein aging mechanisms, Creative Biolabs provides the analytical clarity you need. Let's simplify the complexity of glycation and AGEs—contact our experts today and accelerate your glycoprotein research with confidence.
References
-
Twarda-Clapa, Aleksandra, et al. "Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs." Cells 11.8 (2022): 1312. https://doi.org/10.3390/cells11081312
-
Rungratanawanich, Wiramon, et al. "Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury." Experimental & molecular medicine 53.2 (2021): 168-188. https://doi.org/10.1038/s12276-021-00561-7
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 AGE formation via glycation.1,3
Fig.1 AGE formation via glycation.1,3
 Fig.2 AGE accumulation in aging and alcohol-induced organ damage.2,3
Fig.2 AGE accumulation in aging and alcohol-induced organ damage.2,3

