Glycosylation Research
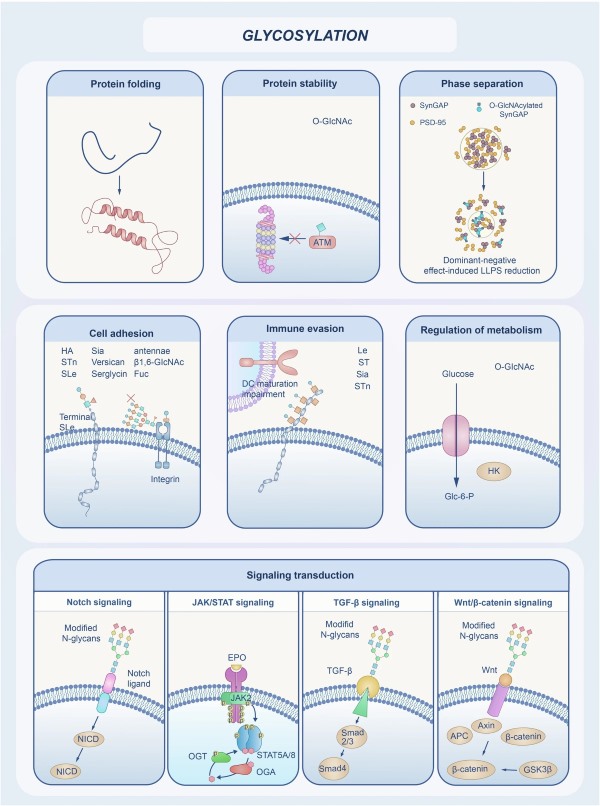 Fig.1 The diverse biological functions of glycosylation.
Fig.1 The diverse biological functions of glycosylation.
For many years, biological research centered on the flow of information from DNA to RNA to proteins. But as our technologies and understanding expanded, we uncovered an entirely new layer of biological control, an intricate system of information conveyed by sugars. This is the domain of glycobiology. Glycosylation, the precise, enzyme-directed process of attaching glycans to proteins and lipids, is one of the most complex and critically important post-translational modifications (PTMs) in all of biology. The protein glycosylation status of a molecule is fundamental, playing crucial roles in key physiological processes, including cell-cell interaction, immune recognition, receptor signaling, and biomolecular clearance. It dictates how a protein folds, its stability, where it goes in the cell, and how it interacts with other molecules. Ultimately, its entire function depends on it. A protein without its correct glycan profile is simply incomplete. Its function can be compromised entirely or altered in ways that have profound biological consequences. Here at Creative Biolabs, we are genuinely excited to explore the world of glycosylation research with you.
Before we can explore the applications, we must have a solid grasp of the "what" and "how." Glycosylation is not one single action; it's a vast collection of processes. They occur in different cellular locations, mainly the endoplasmic reticulum (ER) and the Golgi apparatus. The level of complexity here is truly incredible. Think about this: protein synthesis is template-driven by mRNA. Glycan synthesis is not. It depends entirely on the sequential action of hundreds of specialized enzymes (glycosyltransferases and glycosidases). The expression and location of these enzymes can change depending on the cell's type, age, and environment. This creates a spectacular amount of diversity, a phenomenon we call microheterogeneity, where a single protein attachment site can feature hundreds of different glycan structures.
The two most extensively studied forms of protein glycosylation are N-linked and O-linked. The most studied of these is N-glycosylation, which begins in the ER when a large, pre-assembled oligosaccharide block is attached to an asparagine (Asn) residue. This attachment is highly specific, occurring only within the sequence Asn-X-Ser/Thr. This initial glycan is then extensively modified as the protein moves through the ER and Golgi, resulting in high-mannose, complex, or hybrid structures. This N-glycosylation process is crucial for ensuring correct protein folding and quality control.
Equally important, though far more diverse, is O-glycosylation. The most common form, mucin-type O-glycosylation, involves attaching N-acetylgalactosamine (GalNAc) directly to a serine or threonine residue. Unlike its N-linked counterpart, there is no simple consensus sequence, making its prediction exceptionally difficult. This process, which primarily occurs in the Golgi, builds the glycan one sugar at a time, resulting in a vast array of structures. Other forms, like O-GlcNAcylation, occur in the cytoplasm and nucleus, acting as a crucial regulator of signaling pathways, much like phosphorylation.
Understanding these foundational pathways is the first step toward analyzing or engineering them. We have compiled a comprehensive exploration of these complex pathways in our guide, "What is Glycosylation."
The Challenge of Analysis: Viewing the Glycome
Because glycosylation creates such complex and heterogeneous results, its analysis has long been a significant bottleneck for researchers. This is the precise challenge that the field of glycoproteomics was created to solve. Glycoproteomics is the large-scale identification, characterization, and quantification of glycoproteins and their attached glycan structures. This is a challenging task, as glycans' properties—being polar, branched, and isomeric—make them very hard to analyze with standard proteomics methods.
A high-quality glycosylation analysis workflow, such as the one we've perfected at Creative Biolabs, is a sophisticated process. It begins with meticulous sample preparation and protein extraction from tissues, cells, or biofluids. Following this, the proteins are digested (typically with trypsin) into smaller peptides, some of which—the glycopeptides—still have their glycans attached. Perhaps the most critical step is next: glycopeptide enrichment. Because glycopeptides are often present in low abundance, we must employ powerful techniques such as lectin affinity chromatography or HILIC to isolate them selectively. Once isolated, these glycopeptides are introduced to high-resolution mass spectrometers (MS) for precise mass measurement. Finally, this complex fragmentation data requires specialized data analysis software to identify the peptide sequence, determine the glycan composition, and pinpoint the exact site of attachment. This end-to-end analytical pipeline provides the answers to the most critical questions: Which proteins are glycosylated? Where are the modification sites? What is the specific structure of the glycan at each site? And how does this entire "glycome" change in disease or after treatment? The insights gained from robust glycosylation analysis are the fundamental support for all other glycosylation research.
Glycosylation Research Areas
For a long time, changes in glycosylation observed in diseases were considered merely a side effect of the pathology. We now know this is absolutely incorrect. Aberrant glycosylation is often a primary cause or major contributor to disease progression, especially in cancer. Cancer cells fundamentally alter their metabolic and enzymatic systems, which leads to dramatic shifts in glycan synthesis pathways. These changes manifest in several ways. For instance, tumor cells often display truncated, incomplete O-glycosylation structures, such as the Tn and sialyl-Tn (sTn) antigens, which are strongly associated with metastatic potential. At the same time, many cancers exhibit hypersialylation, the addition of sialic acid residues to the ends of glycan chains. This creates a strong negative charge on the cell surface, which can promote repulsion from immune cells and facilitate the detachment of cancer cells from the primary tumor. Furthermore, altered N-glycosylation branching is directly linked to increased cell migration, invasion, and resistance to chemotherapy.
Cancer is just one powerful example. Congenital disorders of glycosylation (CDGs) are genetic diseases caused by mutations in enzymes involved in glycosylation, resulting in severe, multisystemic symptoms. Even in neurodegenerative diseases like Alzheimer's, aberrant O-glycosylation in the brain is thought to disrupt normal protein signaling, contributing to toxic protein aggregation. Uncovering these connections opens an incredibly valuable new window for diagnostics and therapeutics. This pivotal area of modern medicine is the specific focus of our detailed insights on Glycosylation in Disease Research .
The immune system relies on a complex and constant series of molecular interactions. Many of these interactions are directly governed by glycosylation. A notable example is the therapeutic antibody, specifically immunoglobulin G (IgG). An IgG antibody has a crucial N-glycosylation site on its Fc region. The specific glycan structure at this site functions as a molecular control point, dictating the antibody's effector function. A glycan with fucose (fucosylated) has a lower affinity for the FcγRIIIa receptor on Natural Killer (NK) cells. A glycan lacking fucose (afucosylated) binds this receptor with much higher affinity, dramatically increasing the antibody's power to trigger Antibody-Dependent Cell-mediated Cytotoxicity (ADCC). This discovery is precisely why "glyco-engineering" has become such a critical field in biotherapeutics.
Beyond antibodies, glycosylation is everywhere in immunology. It is fundamental to immune cell trafficking, where selectin proteins mediate the movement of leukocytes along blood vessel walls to reach infection sites. It's also the basis for pathogen recognition, as innate immune receptors are specifically structured to recognize foreign glycan patterns on bacteria and viruses, triggering the immune response. Even the T-cell receptor itself is heavily glycosylated, and these glycans modify the sensitivity and duration of T-cell signaling. This interplay is a vital factor in both health and therapeutic design, a topic we explore thoroughly in our resources on Glycosylation in Immunology .
Viruses are masters of adaptation, having learned to exploit the host cell's glycosylation machinery for their own survival. Many of the most significant enveloped viruses, including HIV, Influenza, and coronaviruses, have surface proteins that are extensively coated in host-derived glycans. This dense layer serves a dual purpose. Critically, it provides immune evasion, acting as a protective layer that hides the foreign viral proteins from the host immune system and blocks neutralizing antibodies. It also ensures infectivity, as viruses hijack the host's N glycosylation pathway to ensure their own envelope proteins are folded and processed correctly. Without this, the resulting viral particles are often non-infectious.
Furthermore, many viruses use host cell glycans as their first point of contact. Influenza's hemagglutinin protein binds to sialic acid residues on respiratory cells. The SARS-CoV-2 spike protein, while binding to the ACE2 protein, is also profoundly modulated in terms of stability and function by its extensive protein glycosylation. This makes viral glycosylation an exciting target for antiviral therapies. We cover the critical topic of viral adaptation and therapeutic opportunities in our dedicated summary, Glycosylation in Virus Research .
Cell surface receptors are the cell's primary sensors for the external environment, detecting signals and transmitting them to the interior. The function of these receptors is exquisitely sensitive to glycosylation.
Nearly all cell surface receptors are glycoproteins, and the N-glycans on them are vital for multiple functions. They are essential for initial folding and trafficking; if N glycosylation is blocked in the ER, the receptor may never reach the cell surface. They also directly influence ligand binding, as the glycan itself can be part of the binding pocket or can change the pocket's shape. Moreover, glycans can physically promote or hinder receptor dimerization, a critical step for activation. Finally, they can interact with glycan-binding proteins to organize receptors into specific areas on the cell membrane, concentrating them for more efficient signaling. Growth factor receptors, such as EGFR, are a prime example, as changes in their glycosylation are known to affect signaling and contribute to uncontrolled cell growth. How these glycans control the flow of information is the central subject of our overview on Glycosylation in Receptor Biology .
If we want to control the final glycosylation profile—for instance, to engineer a better therapeutic or understand a disease—we must look beyond the final product. We must understand the process. This is the exciting and powerful goal of Glycosylation Pathway Analysis. This field investigates the entire cellular synthesis process—the complete network of enzymes, transporters, and substrates in the ER and Golgi that work together to perform glycan synthesis. This investigation employs a multi-omics approach, integrating transcriptomics to measure the mRNA levels of key enzymes, proteomics to quantify their expression and localization, and metabolomics or flux analysis to track the flow of sugar precursors that supply the building blocks for glycosylation.
By building a comprehensive model of this pathway, we can identify critical control points or bottlenecks that may hinder its progress. For example, in a CHO cell line, we might discover that a specific enzyme is underexpressed, leading to an undesirable glycan structure on the final antibody product. Glycosylation pathway analysis is the key to rational "glyco-engineering." It allows us to move beyond chance, enabling us to precisely edit the cellular machinery to produce a specific, defined, and optimal glycoform. To truly engineer the output, you must first map the entire process, which is the core principle of our approach to Glycosylation Pathway Analysis .
Why Custom Glycosylation Matters
At Creative Biolabs, we offer a comprehensive service suite to help you achieve your glycoprotein objectives effectively, using our combined expertise in glyco-analytics, glyco-engineering, and glyco-testing. Our service is straightforward: we partner with you to understand your glycoprotein challenge, design the appropriate workflow, and deliver actionable results that facilitate your research. Here are our Custom Glycosylation Services :
-
Glycosylation Analysis Services : This is the foundation: "What do you have?" Our advanced analytical team precisely identifies, characterizes, and quantifies the complex glycan structures on your molecule. We map N-linked and O-linked sites, determine structural details, and deliver a complete, high-resolution profile of your glycoprotein.
-
Glycosylation Engineering Platform : This is the design phase: "What do you need?" Our platform moves beyond analysis to active creation. We enable the rational engineering of glycosylation pathways in various expression systems (like CHO, yeast, or E. coli) to produce specific, optimized glycoforms with your desired therapeutic functions.
-
Glycosylation Testing Services : This is the application: "What does this glycan profile mean?" We connect glycosylation patterns directly to biological and clinical outcomes. This service suite focuses on using glycosylation as a powerful discovery tool, helping you find solutions for complex disorders and identify critical disease indicators.
The complexity of glycosylation is truly immense. But the opportunities it presents—for new diagnostics, more powerful biotherapeutics, and a deeper, more fundamental understanding of life—are even greater. Successfully navigating this field demands an extraordinary combination of deep expertise, advanced technology, and meticulous execution. The challenges are significant, from high-sensitivity glycopeptide enrichment for glycoproteomics to the intricate dissection of enzymatic pathways. Creative Biolabs has served as a dedicated partner to researchers and pharmaceutical companies worldwide. We provide a world-class suite of services in glycosylation analysis, engineering, and custom research. Our team of scientists is proficient in these techniques, and we are also genuinely passionate about advancing the boundaries of glycosylation research.
Your project deserves a partner who understands the nuance and power of this biological system. We invite you to explore these resources, and when you are ready, let's talk. We truly want to hear about your work. Contact our scientific team today to discuss your unique research goals and discover how Creative Biolabs can accelerate your path to discovery.
Reference
-
He, Mengyuan, Xiangxiang Zhou, and Xin Wang. "Glycosylation: mechanisms, biological functions and clinical implications." Signal Transduction and Targeted Therapy 9.1 (2024): 194. Distributed under Open Access license CC BY 4.0 , without modification. https://doi.org/10.1038/s41392-024-01886-1
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 The diverse biological functions of glycosylation.
Fig.1 The diverse biological functions of glycosylation.

