Intracellular delivery—the targeted transport of therapeutic molecules directly into a cell—presents a complex challenge in modern medicine, where therapeutic efficiency is often hindered by the body's natural defense mechanisms. While membrane-fusion-based internalization offers a superior pathway by bypassing the degradative endo-lysosomal system, it faces a significant obstacle from the protein corona that forms in vivo. This complex problem requires innovative solutions, and the strategic use of lipid-based drug delivery systems is emerging as a promising research area to provide novel ideas for intracellular delivery. As a leading partner in this field, Creative Biolabs is at the forefront, offering specialized solutions to navigate these challenges for our clients.
The promise of intracellular delivery is immense, offering the potential to treat diseases at their genetic and molecular roots. However, for a drug carrier to be successful, it must overcome a series of biological barriers. Chief among these is avoiding the body's natural cellular "waste disposal" system, the endo-lysosomal pathway. Traditional carriers are often internalized through endocytosis and subsequently trapped in the lysosome, where low pH and hydrolytic enzymes degrade the therapeutic payload before it can reach its intended target—the cytoplasm. This renders many drug candidates ineffective, regardless of their intrinsic potency.
Although membrane fusion offers a highly efficient route for direct cytoplasmic delivery, this mechanism is often thwarted in a physiological environment. Upon entering the bloodstream, a nanocarrier is instantly coated by a complex layer of adsorbed plasma proteins, forming what is known as a "protein corona." This corona effectively masks the carrier's engineered surface, preventing its intended interaction with the cell membrane. Instead, the cell recognizes the protein-coated particle as a foreign invader, marking it for clearance via endocytosis. This process inevitably leads to lysosomal entrapment and degradation of the therapeutic payload. This challenge highlights the urgent need for next-generation carriers, which possess stealth properties and can actively resist protein adsorption.
Groundbreaking research from Nature Communications introduces a definitive solution: antifouling liposomes engineered for effective in vivo delivery. This strategic design directly addresses the protein corona problem through precise lipid engineering. The key is a specialized lipid composition, rich in zwitterionic phosphorylcholine groups, which creates a surface that actively resists protein adsorption. By forming a strong hydration layer, these groups provide a steric and electrostatic defense that prevents plasma proteins from adhering, thereby preserving the liposome's function and enabling it to reach its target.
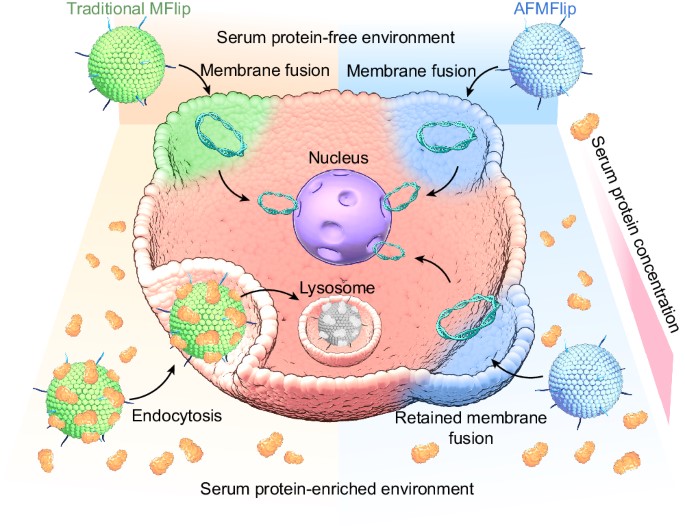 Fig. 1 Traditional membrane fusion liposomes (MFlips) and antifouling MFlips (AFMFlips).1
Fig. 1 Traditional membrane fusion liposomes (MFlips) and antifouling MFlips (AFMFlips).1
The study meticulously detailed the preparation of AFMFlips. Through a specific lipid ratio, the researchers optimized the surface with a high density of zwitterionic phosphorylcholine groups, creating the crucial protein-repellent antifouling property necessary for the delivery system's success.
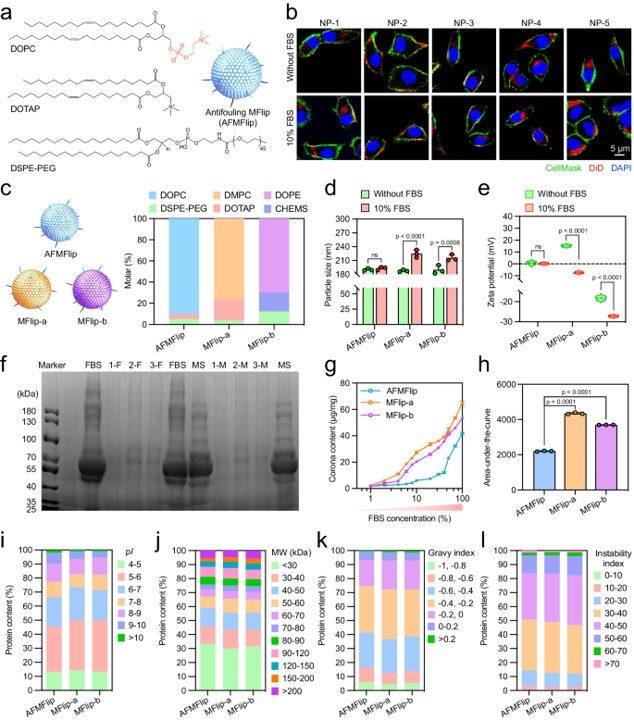 Fig. 2 Preparation of AFMFlips and their anti-protein adsorption effects.1
Fig. 2 Preparation of AFMFlips and their anti-protein adsorption effects.1
To understand the mechanism, researchers observed the liposomes in protein-rich media. Unlike traditional systems that were cleared via endocytosis, AFMFlips demonstrated a membrane-fusion-based pathway. This critical finding confirmed the liposome's ability to bypass lysosomal entrapment and deliver its cargo directly into the cytoplasm.
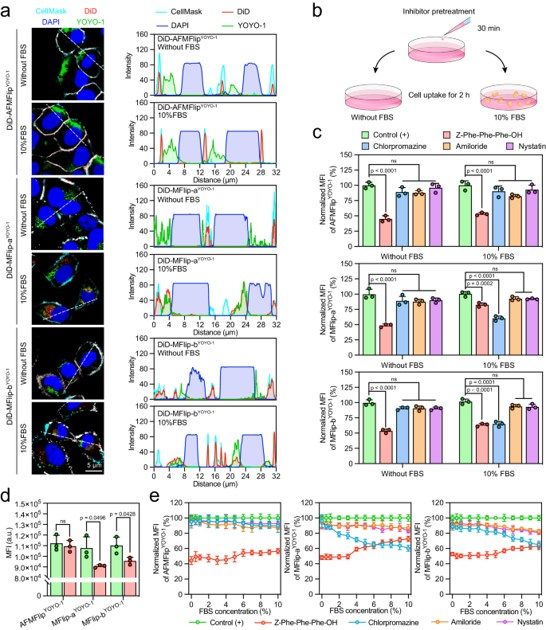 Fig. 3 The cellular internalization of AFMFlips in a protein-rich environment.1
Fig. 3 The cellular internalization of AFMFlips in a protein-rich environment.1
In animal studies, the AFMFlips were shown to maintain their membrane-fusion ability in the complex physiological environment. This resistance to protein fouling and the subsequent successful intracellular transportation of the liposomal payload provides compelling evidence of the system's viability as an effective in vivo delivery tool.
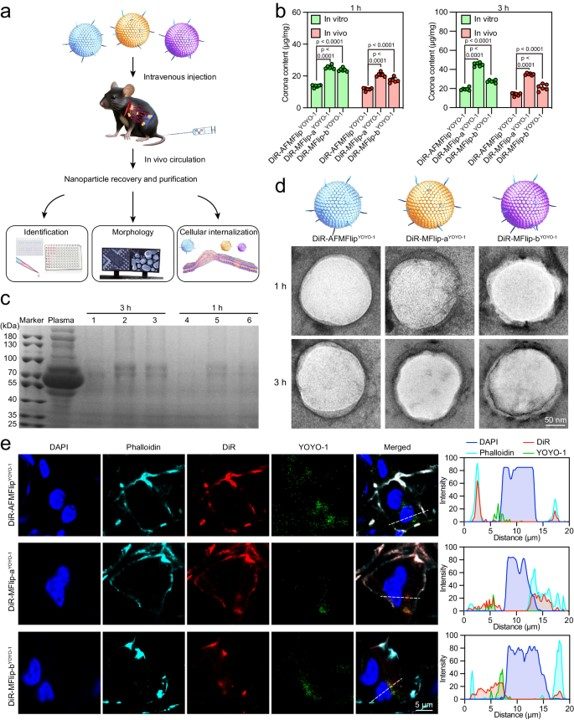 Fig. 4 Anti-protein adsorption and intracellular transport of AFMFlips in C57BL/6 mice.1
Fig. 4 Anti-protein adsorption and intracellular transport of AFMFlips in C57BL/6 mice.1
The platform's efficacy was further validated through its ability to achieve efficient in vivo luciferase transfection. This successful gene delivery, alongside the demonstration of enhanced gene-editing-mediated viral inhibition, confirms the AFMFlips' therapeutic potential as a powerful tool for next-generation nanomedicines.
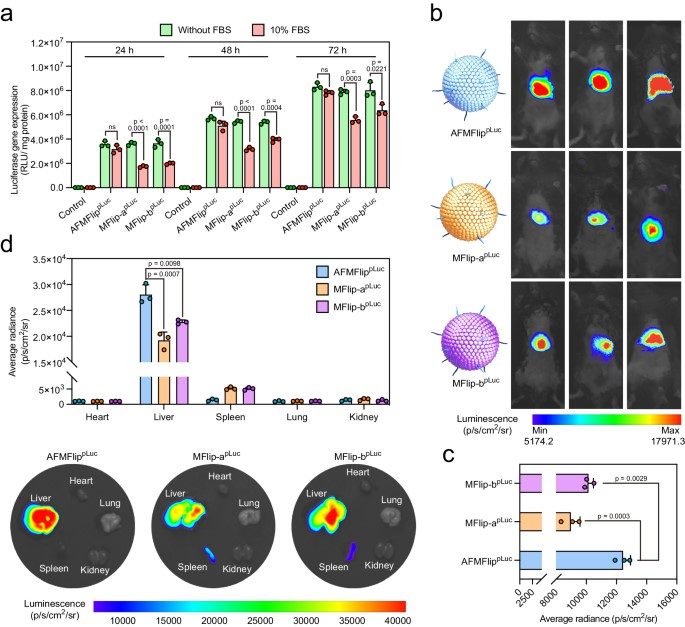 Fig. 5 The in vivo luciferase transfection effect of AFMFlips.1
Fig. 5 The in vivo luciferase transfection effect of AFMFlips.1
The research into AFMFlips provides a strong foundation for future advancements. We can help you leverage these insights to develop new customized liposomes. Contact our experts to learn how Creative Biolabs can support your projects, from optimizing lipid formulas to studying gene transfection, and help you achieve breakthroughs in intracellular drug delivery.
Creative Biolabs offers specialized services to accelerate research and development in antifouling liposomes and other advanced lipid-based drug delivery systems.
| Services/Products | Description | Inquiry |
|---|---|---|
| Customized Liposomes | Similar products can be developed based on the literature to assist customers in achieving breakthroughs. | Inquiry |
| Formula Optimization | Can achieve optimization of various lipid formulas for customers, including components, proportions, etc., such as DSPE-PEG, DOPC, DOTAP. | Inquiry |
| Fluorescent Staining | We offer customized fluorescent-labeled liposomes, such as DID dyes. You can also purchase our fluorescent dyes to treat your liposomes, thereby achieving staining. | Inquiry |
| Membrane Fusion | Helps you study membrane fusion situations. | Inquiry |
| Gene Transfection | We offer ready-to-use Fluc mRNA-LNP (Cat: LDLY-0325-LD505), which can be used for control experiments and assist customers in conducting rapid research during cell transfection. | Inquiry |
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry