Lipid-based drug delivery systems, such as liposomes, have revolutionized how therapeutics are formulated and administered. These nanoscale vesicles offer an elegant solution for encapsulating a wide range of drugs, from small molecules to complex nucleic acids, protecting them from degradation and enabling targeted delivery. A key component in many of these formulations is cholesterol (Chol), which plays a pivotal role in ensuring the structural integrity and functionality of the lipid bilayer. Creative Biolabs is at the forefront of this field, providing expertise and innovative solutions that transform research ideas into viable and effective products for your drug delivery research.
The incorporation of cholesterol into liposomal membranes is a cornerstone strategy for achieving drug carrier stability. It is essential for modulating membrane fluidity, enhancing rigidity, and creating a robust barrier that minimizes permeability. By optimizing lipid packing, cholesterol effectively prevents payload leakage, ensuring the vesicle remains intact during its journey to the target site.
Despite its indispensable role in formulation, conventional cholesterol becomes a critical vulnerability upon in vivo administration. The body's own high-density lipoproteins (HDL) systematically extract cholesterol from the lipid bilayer, destabilizing the very structure it was meant to fortify. This rapid depletion triggers premature drug release, severely compromising therapeutic outcomes. Addressing this inherent weakness is therefore not just an optimization, but a fundamental prerequisite for the clinical success of liposomal drug delivery.
Addressing the critical challenge of cholesterol extraction, recent scientific research has focused on a novel solution: the covalent modification of lipids. The core hypothesis is that by chemically linking cholesterol to a membrane-forming lipid, it would retain its stabilizing effects while being prevented from leaving the liposome. This approach aims to create a more robust and stable drug carrier, minimizing premature payload release and enhancing therapeutic efficacy.
The potential of cholesterol-modified lipid bilayers lies in their ability to overcome a fundamental limitation of traditional liposome formulations. By chemically anchoring cholesterol to the lipid backbone, researchers are creating a more robust and stable nanocarrier. This structural modification has wide-ranging implications, from improving in vivo stability and circulation time to enhancing the overall therapeutic index of encapsulated drugs.
Researchers systematically synthesized various SM-Chol conjugates with different linkers to identify the most stable formulation. Through techniques like Differential Scanning Calorimetry (DSC) and Atomic Force Microscopy (AFM), they confirmed that the new lipids retained cholesterol's ability to condense the membrane, and the disulfide-bonded conjugate with a longer linker exhibited superior rigidity and stability.
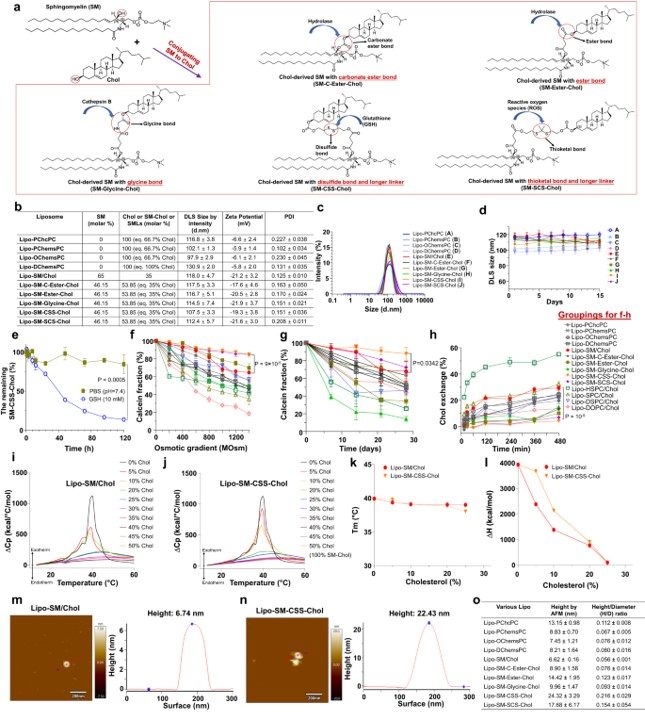 Fig. 1 Development of SM-derived Chol Liposome (Lipo-SM-Cho).1
Fig. 1 Development of SM-derived Chol Liposome (Lipo-SM-Cho).1
In animal models, the improved stability of SM-Chol-based liposomes translated into significantly enhanced pharmacokinetics. The liposomes demonstrated longer circulation times and improved tumor accumulation compared to traditional formulations. This evidence highlighted the platform's potential for passive targeting and efficient drug delivery to cancerous tissues.
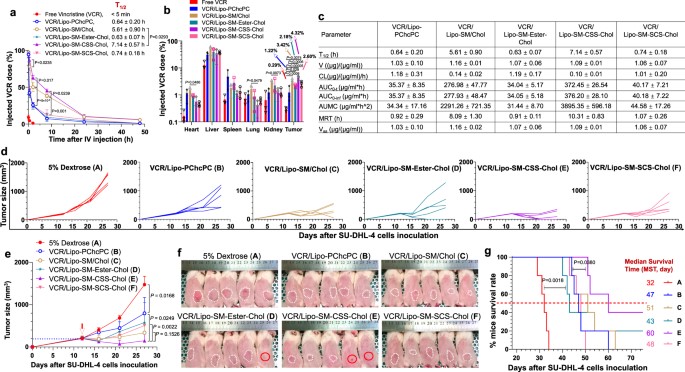 Fig. 2 VCR/Lipo-SM-CSS-Chol improves circulation time, tumor delivery and therapeutic effect.1
Fig. 2 VCR/Lipo-SM-CSS-Chol improves circulation time, tumor delivery and therapeutic effect.1
The SM-Chol system improved the antitumor efficacy of several drugs, including vincristine (VCR), irinotecan (IRI), and doxorubicin (DOX), in various cancer models. Crucially, the formulation also increased the maximum tolerated dose of the drugs while reducing systemic toxicities. This demonstrates that the enhanced stability not only improves efficacy but also provides a safer therapeutic profile.
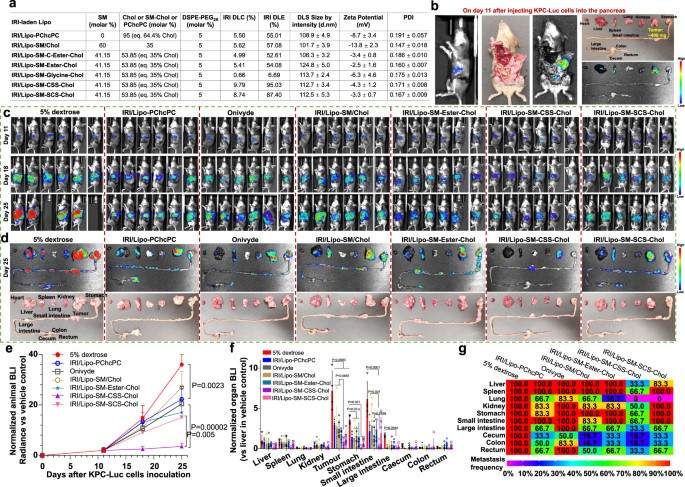 Fig. 3 Lipo-SM-CSS-Chol can enhance the efficacy of IRI in the treatment of PDAC. 1
Fig. 3 Lipo-SM-CSS-Chol can enhance the efficacy of IRI in the treatment of PDAC. 1
These scientific findings underscore the importance of innovating beyond conventional lipid formulations. The development of a more stable, universal lipid platform through covalent modification is a significant step forward. Creative Biolabs is well-positioned to help you explore and implement this technology. Contact us to discuss how our expertise can accelerate your research in this area.
Creative Biolabs offers specialized services to help you navigate the complexities of cholesterol-modified lipid bilayer development.
| Services/Products | Description | Inquiry |
|---|---|---|
| Conjugate | We specialize in advanced conjugate strategies, including the covalent conjugation of lipids to create novel, stable formulations like the SM-Chol platform discussed in the literature. | Inquiry |
| Nucleic Acid Synthesis | Our services include the synthesis of various nucleic acids, such as mRNA, siRNA, and their optimized delivery via advanced lipid-based systems, building on the therapeutic applications highlighted in recent research. | Inquiry |
| Liposome Customization | We offer comprehensive liposome customization, allowing you to adjust lipid composition, ratios, preparation methods, and particle size. We can also provide services like calcein loading for visualization to support your research needs. | Inquiry |
| LNP Customization | Our platform provides a variety of selectable ionizable and targeted lipids, allowing us to help you customize Lipid Nanoparticles (LNPs). We can also adjust the N/P value to optimize your specific formulation. | Inquiry |
| Complete Detection Platform | In addition to basic characterization, our platform features advanced technologies like Differential Scanning Calorimetry (DSC) and Cryo-Electron Microscopy (Cryo-EM) to provide a deeper understanding of your liposome and LNP formulations. | Inquiry |
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry