Liposomes have long been established as a leading modality in nanomedicine, offering a sophisticated platform for the targeted delivery of a broad spectrum of therapeutics. Their unique structure as lipid-bilayer vesicles enables the encapsulation and stabilization of both hydrophilic and hydrophobic drug molecules, resulting in improved pharmacological profiles and reduced off-target effects. Despite this immense potential, a persistent challenge has been the successful commercial translation of liposomal formulations, which has been largely impeded by limitations in scalable, robust, and economically viable manufacturing and purification processes. At Creative Biolabs, our core mission is to address these translational barriers. We harness our specialized knowledge in lipid-based delivery systems to develop advanced, scalable technologies.
Conventional liposome manufacturing methods, such as thin-film hydration followed by sonication or extrusion, are often time-consuming and labor-intensive. These batch-based processes are difficult to scale up, and they frequently suffer from poor reproducibility, inconsistent particle size distribution (polydispersity), and low encapsulation efficiency. Furthermore, a critical and often lengthy step is the purification of the final product to remove non-encapsulated drug, residual organic solvents, and other contaminants. Historically, this has involved methods like dialysis or size-exclusion chromatography, which can be inefficient and lead to significant product loss, further diminishing the economic feasibility of new liposomal nanomedicines.
The need for a more robust and streamlined approach is clear: to accelerate the clinical translation of liposome-based drugs, we must develop manufacturing processes that are not only cost-effective but also capable of producing high-quality, uniform nanoparticles at a commercial scale.
A key advancement in addressing these production challenges has been the development of continuous-flow microfluidic systems. This "lab-on-chip" technology offers a paradigm shift from traditional batch processes by providing exquisite control over the fluid flow and mixing. The ability to precisely dictate the conditions of liposome self-assembly in a confined microenvironment results in the production of highly uniform vesicles with a narrow size distribution, a crucial factor for clinical performance and regulatory approval. The ability to control parameters like lipid composition, size, and charge with high reproducibility is a game-changer for formulation development. These advancements lay the groundwork for a more efficient and scalable future for liposome manufacturing.
A study published in Scientific Reports presented a novel module-based microfluidic system that offers a highly effective and rapid solution for liposome production and purification. This system, which integrates both manufacturing and downstream processing, demonstrates how modern technology can bridge the gap from bench-top research to scalable production.
The study's goal was to overcome multi-step batch limitations by integrating liposome preparation, modification, and purification into one continuous process. A module-based microfluidic system with a Staggered Herringbone Mixer (SHM) for self-assembly and a tangential flow filtration (TFF) device for purification was developed. Results showed the system produced uniform vesicles (below 300nm) with controlled attributes, demonstrating high reproducibility.
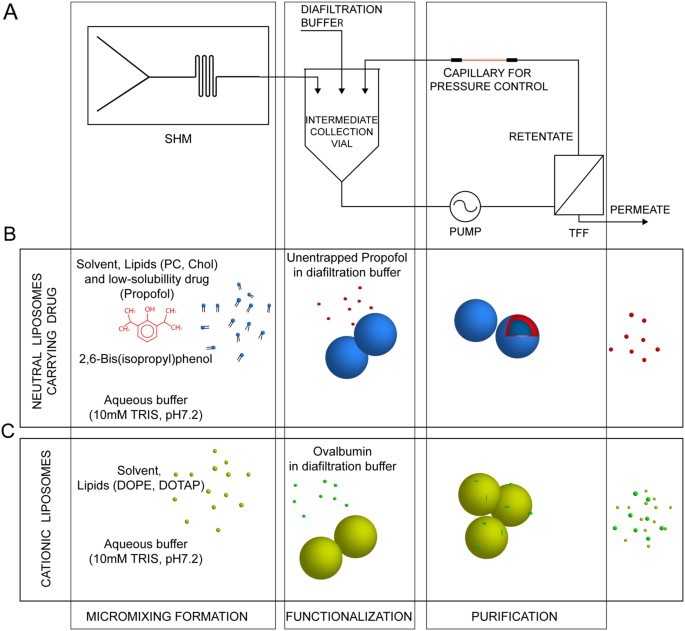 Fig. 1 Schematic overview of the module-based microfluidic system.1
Fig. 1 Schematic overview of the module-based microfluidic system.1
This experiment aimed to validate the integrated purification step. The TFF device was tested with model contaminants: a drug (>95% propofol reduction), a protein (>90% ovalbumin reduction), and an organic solvent (>95% ethanol reduction). The exceptional results in under four minutes confirm this rapid and effective purification as a significant advantage over traditional, time-intensive methods.
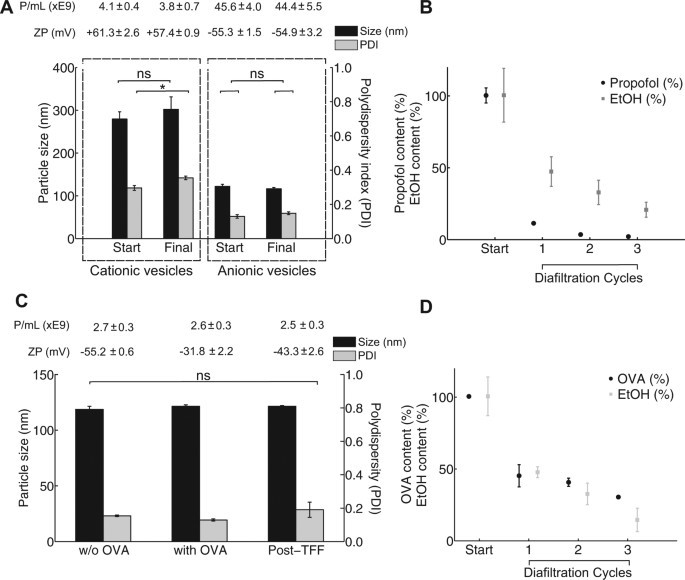 Fig. 2 The effect of filtration on particle characteristics of cationic and anionic liposomes.1
Fig. 2 The effect of filtration on particle characteristics of cationic and anionic liposomes.1
The study showcased the system's potential for scalability and use as a high-throughput process development tool. The modular, bench-top equipment's flexible operating conditions allow for both rapid formulation screening and higher-volume production with longer run times. This confirmed a direct and accelerated path from lab-scale to industrial application.
Ready to advance your liposome project? Our expert team is ready to provide the scientific guidance and technical solutions you need to overcome your development hurdles. From initial concept to process optimization, we are your trusted partner in lipid-based drug delivery. Contact us today to discuss how we can support your specific project needs in scalable liposome manufacturing and help you bring your nanomedicine to market faster.
Creative Biolabs offers specialized services to accelerate your research and development in scalable liposome manufacturing and lipid-based drug delivery systems. Our experts are ready to assist with every stage of your project.
| Services/Products | Description | Inquiry |
|---|---|---|
| Formulation Development | We can help you design and optimize liposomal formulations using cutting-edge microfluidic technologies to achieve the desired particle size, encapsulation efficiency, and stability. | Inquiry |
| Process Optimization | Development of robust, scalable, and cost-effective manufacturing and purification processes to ensure batch-to-batch consistency. | Inquiry |
| Advanced Characterization | We provide state-of-the-art analytical services to meticulously characterize your nanoplatform, from particle size analysis to zeta potential measurement, ensuring the data is robust and reliable. | Inquiry |
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry