While immunotherapy has revolutionized oncology, its efficacy is often constrained by the formidable, immunosuppressive tumor microenvironment. Conventional drug delivery systems frequently fail to penetrate these complex biological barriers, creating a critical need for smarter, more targeted therapeutic strategies. In response, lipid-based delivery systems—especially those with triggered-release capabilities—have emerged as a leading solution. Among these, temperature-sensitive liposomes (TSLs) represent a pinnacle of innovation, offering an exquisitely precise method for the on-demand release of therapeutic agents directly at the disease site. As a leading solution in the field of lipid-based drug delivery systems, Creative Biolabs specializes in providing innovative research ideas and development services that empower our clients to precisely target these critical areas in cancer immunotherapy.
TSLs are advanced lipid nanoparticles engineered for precise, heat-activated drug delivery. Their specially formulated membrane is stable at 37 °C but undergoes a rapid phase transition at mildly elevated temperatures (40-42 °C), triggering the release of their cargo.
The mechanism operates in three key stages:
This spatiotemporally controlled release mechanism ensures high drug concentrations at the target site while minimizing systemic toxicity, thereby enhancing both the safety and efficacy of the treatment.
The immune system's remarkable ability to fight disease is a cornerstone of modern medicine. However, many tumors have developed sophisticated mechanisms to evade immune surveillance, creating an immunosuppressive microenvironment. This includes a network of inhibitory cells and factors that suppress the activity of T and Natural Killer (NK) cells, which are crucial for destroying cancer cells. Overcoming this immune suppression requires innovative delivery systems that can not only target the primary tumor but also modulate the immune landscape to restore anti-tumor immunity.
Recent research highlights a new frontier in TSL technology, moving beyond simple delivery to complex, multi-modal immunotherapy. The following insights, drawn from a recent publication, demonstrate how TSLs can be engineered to sequentially target both the primary tumor and the lymph nodes, leading to a powerful systemic immune response.
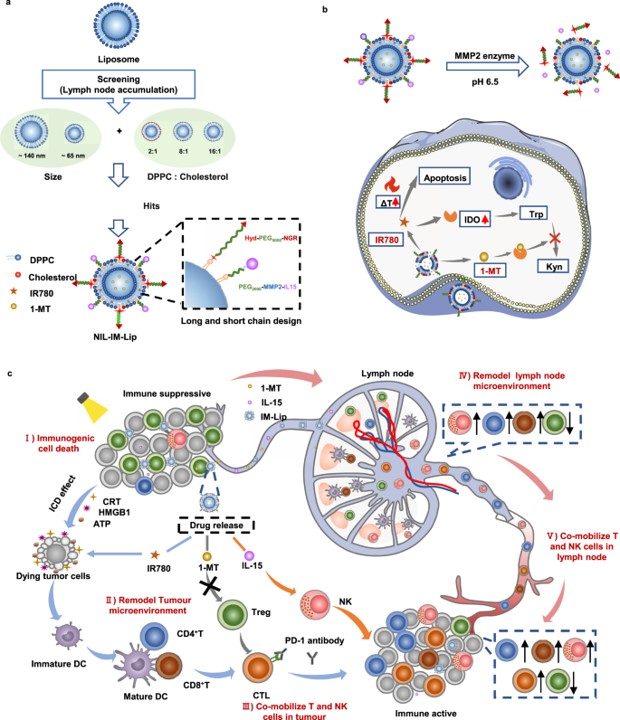 Fig. 1 Nanoinducers reprogramming the TME for T/NK cell mobilization.1
Fig. 1 Nanoinducers reprogramming the TME for T/NK cell mobilization.1
Research explored the efficacy of NIL-IM-Lip with a small size and specific cholesterol ratio to optimize its ability to target lymph nodes, enable xenotype cell delivery, and facilitate efficient photothermal conversion. This meticulous design is key to maximizing therapeutic potential and controlled payload release.
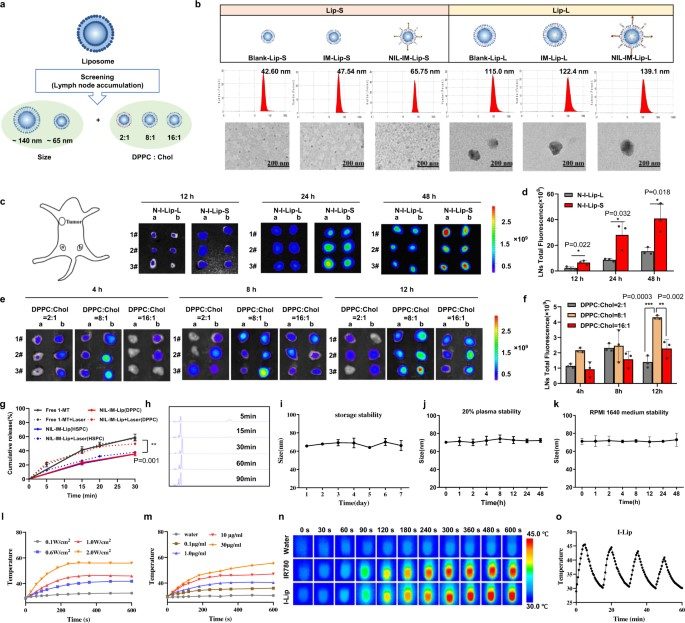 Fig. 2 Optimized NIL-IM-Lip for lymph node targeting, delivery, and photothermal conversion.1
Fig. 2 Optimized NIL-IM-Lip for lymph node targeting, delivery, and photothermal conversion.1
Studies monitored the accumulation of NIL-IM-Lip within tumors and lymph nodes, demonstrating effective delivery to both sites. The research also analyzed the local hyperthermic effects generated by the nanoparticles, confirming their ability to trigger localized drug release and enhance therapeutic outcomes.
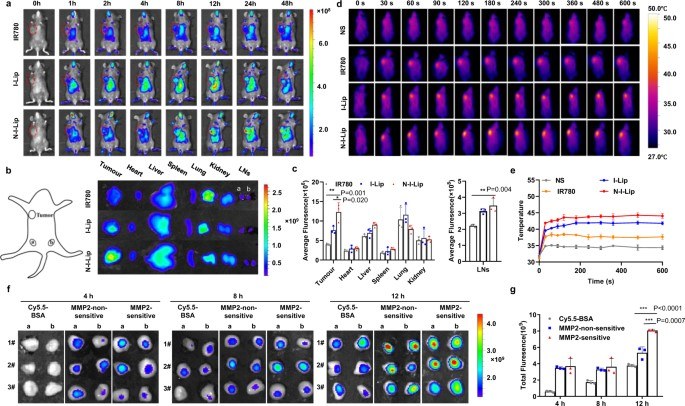 Fig. 3 NIL-IM-Lip accumulation in tumors/LNs and induced hyperthermia.1
Fig. 3 NIL-IM-Lip accumulation in tumors/LNs and induced hyperthermia.1
In laboratory settings, the nanoparticles were shown to induce immunogenic cell death (ICD), promote the maturation of dendritic cells (DCs), and enhance the killing effects of natural killer (NK) cells. These in vitro findings provide strong evidence for the system's ability to initiate a robust anti-tumor immune response.
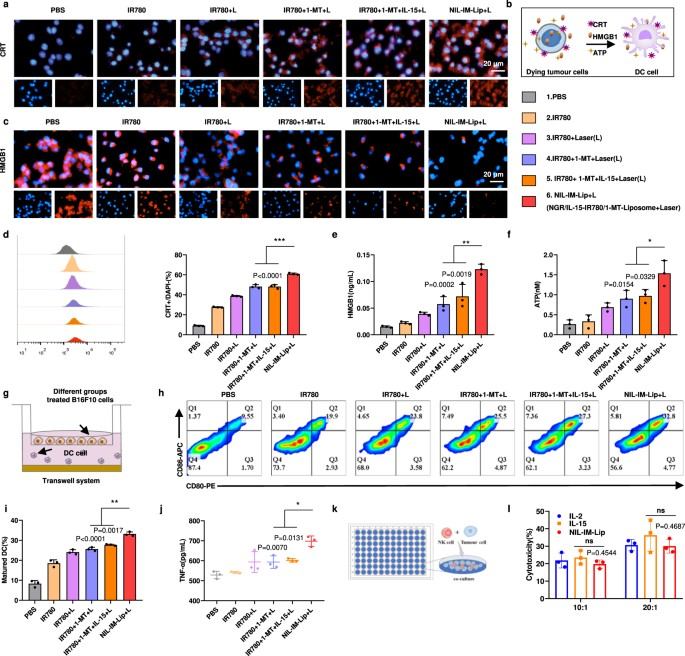 Fig. 4 In vitro ICD induction, DC maturation, and NK cell cytotoxicity by NIL-IM-Lip (Laser).1
Fig. 4 In vitro ICD induction, DC maturation, and NK cell cytotoxicity by NIL-IM-Lip (Laser).1
A key finding was the enhanced anti-tumor effect observed when combining NIL-IM-Lip with a PD-1 monoclonal antibody. This synergistic treatment significantly suppressed tumor growth in both "hot" (B16F10) and "cold" (CT26) tumor models, highlighting the system's broad applicability and potential for complete tumor suppression.
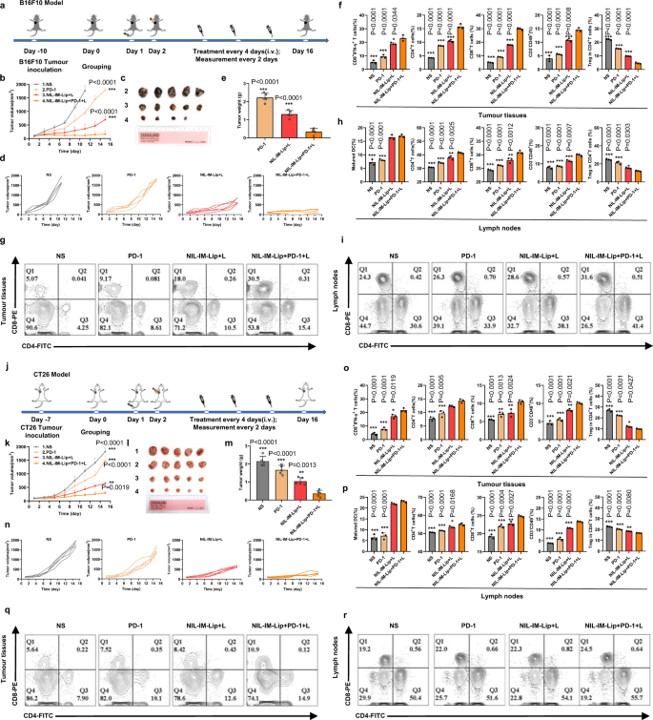 Fig. 5 Synergistic antitumor efficacy of NIL-IM-Lip (Laser) + anti-PD-1 in hot/cold tumors.1
Fig. 5 Synergistic antitumor efficacy of NIL-IM-Lip (Laser) + anti-PD-1 in hot/cold tumors.1
Recent research highlights the power of multi-modal strategies, particularly TSL-based delivery, to overcome complex tumor microenvironments and achieve superior therapeutic outcomes. At Creative Biolabs, we are experts at translating these advanced concepts into practical solutions. We can help you integrate engineered liposomes into your programs to accelerate your R&D goals. Contact us today to discuss how we can support your project.
Creative Biolabs offers a range of specialized services to support your temperature-sensitive liposome and lipid-based drug delivery projects.
| Services/Products | Description | Inquiry |
|---|---|---|
| Particle Size Control | We specialize in the precise control of liposome particle size, a critical factor for biodistribution, stability, and cellular uptake. Our techniques ensure optimal performance for your drug delivery system. | Inquiry |
| Development of Temperature-responsive Liposome | We develop advanced thermosensitive liposomes and other responsive systems triggered by pH, ROS, and more. Our expertise ensures a controlled and targeted release of therapeutic payloads to maximize efficacy and safety. | Inquiry |
| In Vitro Release of Delivery System | Our comprehensive services include detailed in vitro release studies to characterize the behavior of your drug delivery system under various conditions. This data is essential for predicting and optimizing performance. | Inquiry |
| Rich Lipid Library | Our extensive library provides a variety of high-purity lipid raw materials, including DPPC, HSPC, and DSPE-PEG2000-MMP, tailored for lipid-based drug delivery. We also offer custom synthesis for unique lipid requirements. | Inquiry |
| Model Animals | We provide access to a wide range of well-characterized tumor and other disease models, crucial for validating the in vivo efficacy and safety of your drug delivery systems and accelerating preclinical research. | Inquiry |
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry