The global market for bioactive molecules—from therapeutic peptides to advanced cosmetic ingredients—is experiencing unprecedented growth. Among these, collagen stands as a cornerstone protein, revered for its structural integrity and regenerative properties. However, its widespread application in both pharmaceuticals and cosmeceuticals has long been hindered by challenges related to its stability, bioavailability, and susceptibility to degradation. At Creative Biolabs, we specialize in overcoming these formulation hurdles through innovative lipid-based drug delivery systems. The encapsulation of sensitive bioactive compounds within liposomes represents a cutting-edge strategy that not only protects the cargo but also enhances its therapeutic efficacy. A prime example of this innovative approach is the formulation and analysis of recombinant collagen liposomes, which have shown remarkable promise in antioxidative applications.
Traditionally, collagen has been sourced from animal tissues, a process fraught with concerns about immunogenicity, disease transmission, and batch variability. Recombinant humanized collagen (rhCol), a synthetic alternative, circumvents these issues by providing a consistent, high-purity product. The challenge then shifts to delivering this delicate protein to its intended target without losing its biological activity.
Liposomes, as a type of lipid-based drug delivery systems, are ideally suited for this task. These spherical vesicles, composed of one or more lipid bilayers, act as microscopic protective capsules. Their biocompatibility and ability to shield encapsulated molecules from enzymatic degradation and external stress make them a superior delivery vehicle. By combining rhCol with a liposomal carrier, formulators can create a robust delivery system that preserves the collagen's structure and function.
A recent study published in Scientific Reports provides a compelling case study of this process. The researchers focused on optimizing the preparation of recombinant type III humanized collagen-loaded liposomes (rhCol III-LIPS) to maximize encapsulation efficiency—a critical parameter for any drug delivery system.
The study first optimized the preparation of rhCol III-LIPS using specific techniques. The key finding was a high encapsulation rate of 91.89%±0.59% by fine-tuning parameters like the rhCol III-to-lipid ratio. The corresponding chart would visually demonstrate how encapsulation efficiency changes across different formulation conditions, highlighting the optimal ratio for stability.
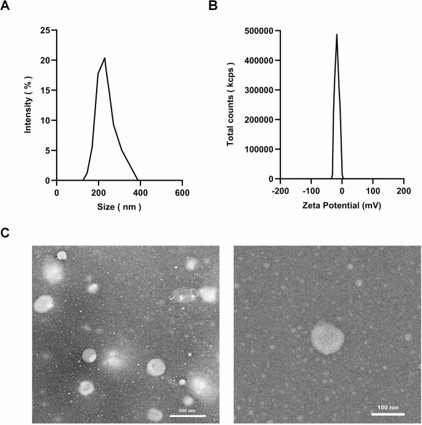 Fig. 1 Physicochemical properties and characterization of rhCol III-LIPS.1
Fig. 1 Physicochemical properties and characterization of rhCol III-LIPS.1
Following formulation, the study analyzed the in vitro antioxidative activity of the rhCol III-LIPS. The high free-radical scavenging rates for DPPH (87.11%), ABTS (93.72%), hydroxyl (86.45%), and superoxide (76.67%) anions demonstrated the formulation's potent protective effects. The accompanying visual data presenting these scavenging rates, providing clear evidence of the formulation's potency.
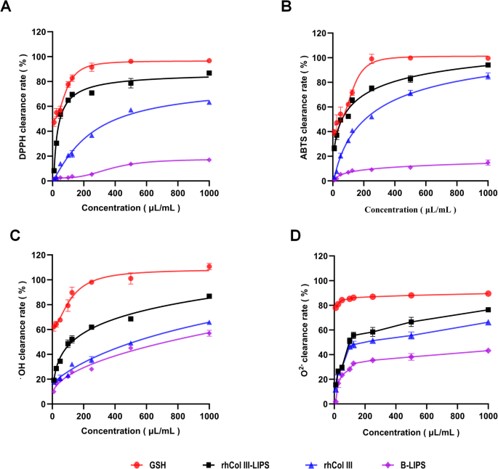 Fig. 2 The scavenging effects of free radicals in each treatment group.1
Fig. 2 The scavenging effects of free radicals in each treatment group.1
Finally, the research investigated the formulation's protective effects against H2O2-induced oxidative injuries in HaCaT cells. The rhCol III-LIPS provided superior protection compared to both un-encapsulated rhCol and blank liposomes. Microscopic images of the cells would show a clear visual difference in viability and health between the control, damaged, and treated groups, proving the formulation's biological efficacy.
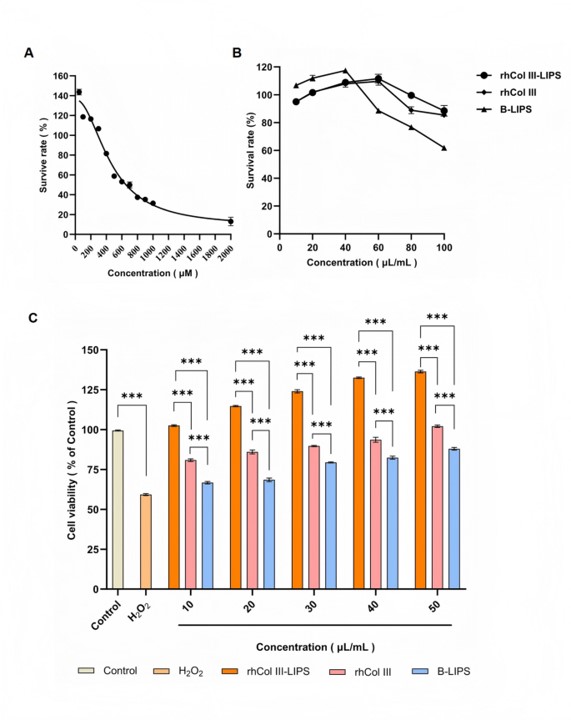 Fig. 3 Effects of each treatment group on the oxidative damage model of HaCaT cells.1
Fig. 3 Effects of each treatment group on the oxidative damage model of HaCaT cells.1
In summary, this research highlights the critical steps from formulation optimization to demonstrating biological efficacy. These results provide a strong scientific basis for using lipid-based systems to enhance recombinant collagen's stability and therapeutic potential. This is precisely where Creative Biolabs' expertise comes in, helping you navigate these complex processes to achieve similar groundbreaking results. Contact us today to discuss how we can apply these insights to your specific project needs.
Creative Biolabs offers a suite of specialized services to support every stage of your project, from initial formulation design to final validation. Our expert team provides comprehensive support tailored to your specific needs in the antioxidative analysis of liposomes and other lipid-based drug delivery systems.
| Services/Products | Description | Inquiry |
|---|---|---|
| Formulation Optimization | We can select the most suitable liposome formulation using advanced methods like response surface methodology, optimizing lipid composition, and proportions to ensure high encapsulation and stability. | Inquiry |
| Comprehensive Characterization Platform | Our platform covers a wide range of evaluations including particle size, PDI, potential, encapsulation rate, and morphology, giving you a complete understanding of your liposome system. | Inquiry |
| Stability Testing | We evaluate the stability of your lipid-based formulations under various environmental conditions, such as different temperatures, to predict shelf life and ensure long-term integrity. | Inquiry |
| In Vitro & In Vivo Antioxidant Effects | Our services utilize extensive cell and animal models to verify the pharmacological effects of your formulations, including antioxidant properties, cellular protection, and more. | Inquiry |
Reference
 For Research Use Only. Not For Clinical Use
For Research Use Only. Not For Clinical UseSupports
Online Inquiry