Glycosylation, a vital post-translational modification of proteins, serves essential roles in many biological processes such as cell recognition, signal transduction, and immune responses. Compared to other modifications like phosphorylation and acetylation, glycosylation has long been considered the "molecular dark matter" of life sciences because of its complex structure and subtle microheterogeneity. Recent advances in chemical synthesis methods, high-sensitivity analytical technologies, and functional research tools have sparked an unprecedented revolution in glycosylation research. Creative Biolabs, as a leader in this field, systematically reviews the latest developments, focusing on three main areas: advances in synthetic chemistry, innovations in analytical techniques, and mechanistic insights into glycosylation functions. We are dedicated to exploring the biological significance of glycosylation in disease, providing researchers with a comprehensive and cutting-edge perspective.
Recent Breakthroughs in Glycosylation Synthetic Chemistry
Traditional carbohydrate synthesis relies heavily on elaborate protecting group strategies, where chemists selectively mask certain hydroxyl groups on sugar molecules to control regioselectivity and stereoselectivity in glycosidic bond formation. Although this method has led to notable achievements, it is inherently limited by its multi-step processes and low efficiency. In 2024, a team developed a catalytic glycosylation platform that enables precise O-glycosylation reactions between minimally protected or even unprotected glycosyl donors and acceptors, successfully overcoming the longstanding challenge of stereocontrolled C–O bond formation amid dense hydroxyl environments. The core mechanism of this breakthrough lies in radical activation of allyl sulfone glycosyl donors to generate highly reactive glycosyl bromide intermediates under mild conditions. These intermediates are then precisely guided by aminoboronic acid catalysts through a network of non-covalent hydrogen bonding and reversible covalent B-O interactions, promoting efficient glycosyl transfer. Remarkably, by modifying the catalyst structure, researchers can flexibly switch glycosylation sites and programmably synthesize challenging 1,2-cis-O-glycosidic linkages.
Using this method, they successfully synthesized otherwise inaccessible glycoforms, including tumor-associated carbohydrate antigens and neuroactive glycan chains. This strategy offers a powerful new tool for glycodrug development, potentially accelerating the innovation of glycan-based vaccines, antibody-drug conjugates (ADCs), and targeted therapeutics. At Creative Biolabs, we harness such cutting-edge advancements to address the complexities of synthesizing biologically relevant glycostructures, we offer comprehensive solutions for your most demanding projects:
-
For complex custom glycoproteins (synthesized via glycoprotein remodeling, native chemical ligation, or other advanced strategies), explore our dedicated capabilities: Custom Glycoprotein Synthesis Services
-
For structurally defined custom glycopeptides (using solid-phase or liquid-phase synthesis) with site-specific modifications, discover our tailored approach: Custom Glycopeptide Synthesis Services
-
For a broad spectrum of carbohydrate synthesis needs–from monosaccharides and oligosaccharides to complex glycoconjugates–rely on our versatile expertise: Custom Glycan Synthesis Services
Advances in Glycosylation Analytical Technologies
Deep Quantitative Glycosylation Analysis (DQGlyco)
Conventional glycoproteomics faces three major limitations: lectin-based enrichment bias, insufficient mass spectrometry (MS) sensitivity, and restricted dynamic quantitative throughput—collectively hindering the resolution of key biological questions. In early 2025, the Savitski group introduced the "Deep Quantitative Glycosylation Analysis (DQGlyco)" platform, marking a transition to a new era of panoramic glycoproteomic profiling. This breakthrough technology employs pH-sensitive covalent capture using phenylboronic acid-modified magnetic beads combined with high-salt lysis buffers to eliminate nucleic acid interference, raising glycopeptide enrichment specificity above 90%. Further, the system leverages the precision separation capability of porous graphitic carbon chromatography to distinguish glycopeptides differing by just one monosaccharide unit. Compared to traditional methods, DQGlyco achieves a 25-fold increase in identification depth, fundamentally transforming our understanding of glycosylation complexity.
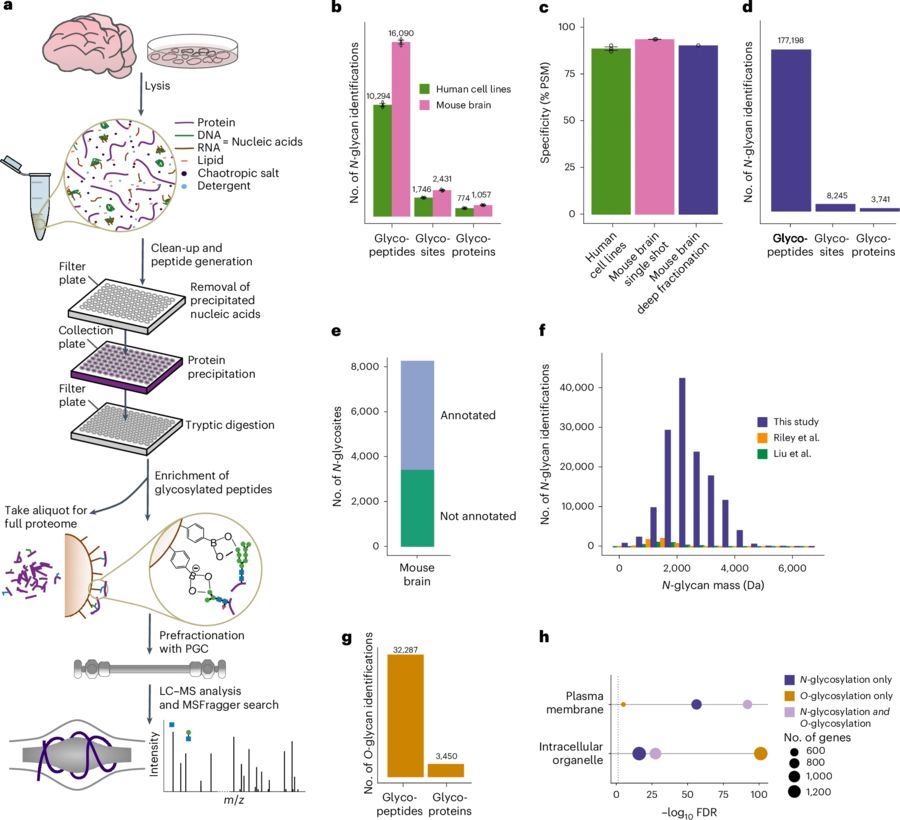 Fig.1 DQGlyco workflow for deep protein glycosylation profiling 1,3
Fig.1 DQGlyco workflow for deep protein glycosylation profiling 1,3
Multidimensional Glycopeptide Enrichment Strategies
To address the coexistence and dynamic variation of multiple glycosylation types (e.g., N-glycosylation, O-GalNAc, O-GlcNAc), researchers developed a novel strategy named HG-TCs (Hydrazide-Gel-based Tandem Chemical Strategies). This approach employs advanced solid-phase materials and bioorthogonal chemistry to simultaneously enrich multiple glycosylation forms. The core innovation lies in immobilizing glycopeptides via azide–alkyne cycloaddition followed by trypsin cleavage to release analytes, forming a single-tube workflow that minimizes sample loss. In application, HG-TCs demonstrated excellent performance in HeLa cell analyses, maintaining high reproducibility even with low-input samples.
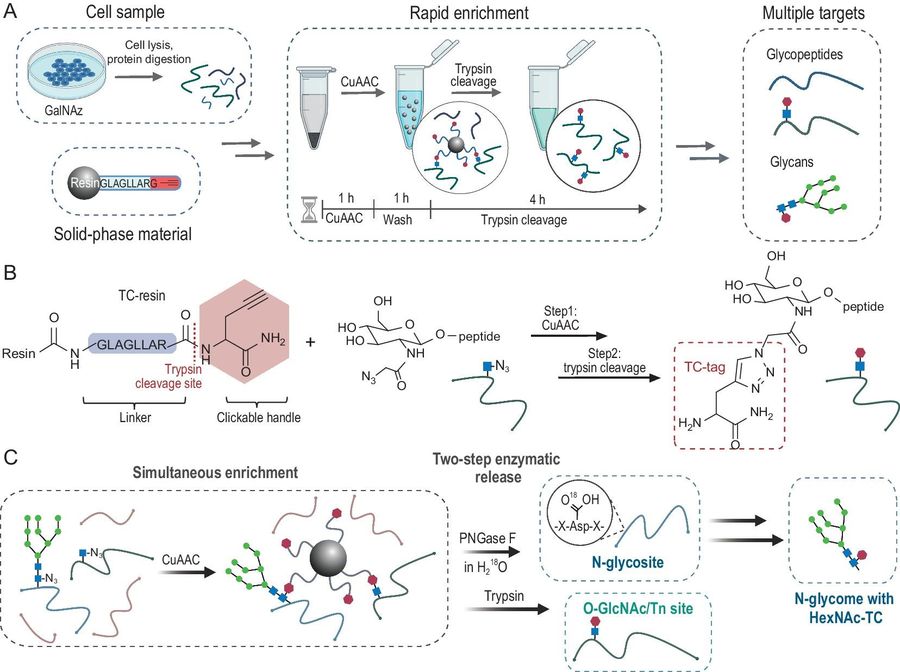 Fig.2 High-throughput enrichment of HexNAc-modified glycopeptides using TC-resins. 2,3
Fig.2 High-throughput enrichment of HexNAc-modified glycopeptides using TC-resins. 2,3
Creative Biolabs' Advanced Technologies for Studying Glycosylation
Creative Biolabs offers an advanced platform for studying glycosylation, providing comprehensive, cutting-edge glycosylation analysis solutions that include but are not limited to:
Glycosylation and Tumor Metastasis
Glycosylation reprogramming during tumor metastasis has emerged as a frontier in cancer biology. A surgical oncology team conducted N-glycosylation profiling on colorectal cancer liver metastasis, uncovering a pivotal role of cathepsin D (CTSD). By analyzing paired primary and liver metastatic samples from 14 patients, they found significantly elevated N-glycosylation at Asn263 (N263) of CTSD in liver metastases, dominated by glycoforms such as H(6)N(2), H(5)N(2), and H(7)N(2). To clarify its functional relevance, the team constructed a glycosylation-deficient CTSD mutant (N263Q). In vitro and in vivo experiments revealed that cancer cells expressing wild-type CTSD formed larger subcutaneous tumors and more prominent liver metastases in nude mice, whereas the N263Q mutant almost completely lost metastatic capability. Mechanistically, N263 glycosylation was essential for correct lysosomal/endosomal trafficking of CTSD. Without this modification, CTSD mislocalized to the cytoplasm, its half-life dropped from over 5 hours to less than 3 hours, and enzymatic activity was markedly reduced. Further investigation revealed that glycosylation-dependent proteolytic activity of CTSD targets mitochondrial medium-chain acyl-CoA dehydrogenase (ACADM)—a key regulator of fatty acid β-oxidation and ferroptosis. Across 90 clinical samples, CTSD expression was inversely correlated with ACADM levels, and patients with low ACADM expression had worse prognoses. These findings highlight a novel mechanism by which glycosylation modulates cell death pathways to facilitate tumor metastasis and suggest a potential therapeutic target for preventing colorectal cancer liver metastasis.
As a globally recognized leader in biologics discovery and development, Creative Biolabs leverages decades of expertise to illuminate glycosylation's biological secrets. Our advanced glycosylation analysis services combine industry-leading platforms—including MS, HPLC, NMR, Glyco-Microarrays—to deliver unprecedented insights into glycoprotein structure-function dynamics for disease research.
References
-
Potel, Clément M., et al. "Uncovering protein glycosylation dynamics and heterogeneity using deep quantitative glycoprofiling (DQGlyco)." Nature Structural & Molecular Biology 32.6 (2025): 1111-1126. https://doi.org/10.1038/s41594-025-01485-w
-
Xiong, Yingying, et al. "Rapid and large-scale glycopeptide enrichment strategy based on chemical ligation." National Science Review 11.11 (2024): nwae341. https://doi.org/10.1093/nsr/nwae341
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 DQGlyco workflow for deep protein glycosylation profiling 1,3
Fig.1 DQGlyco workflow for deep protein glycosylation profiling 1,3
 Fig.2 High-throughput enrichment of HexNAc-modified glycopeptides using TC-resins. 2,3
Fig.2 High-throughput enrichment of HexNAc-modified glycopeptides using TC-resins. 2,3

