Introduction
Glycosylation—the attachment of sugars to proteins or lipids—is far more than a decorative post-translational event. It controls protein folding, trafficking, immunogenicity, and lifespan. Errors in glycosylation are linked to cancer, autoimmune conditions, congenital disorders, and drug inefficacy. At Creative Biolabs, we understand that deciphering glycosylation pathways requires not just powerful tools, but also expert support. Our glycosylation analysis services are designed to help researchers capture the full picture—from early-stage discovery to advanced characterization.
ER and Golgi: Glycosylation's Central Stations
As glycoproteins are synthesized in the ER, co-translational addition of glycans supports proper folding. Creative Biolabs tracks folding status, misfolding, and ER retention using both enzymatic assays and glycoprotein profiling tools. In the Golgi, proteins acquire their final glycan decorations. Creative Biolabs offers advanced glycan modification and labeling services that allow researchers to:
-
Trace specific sugar extensions and caps
-
Introduce functional tags for imaging
-
Monitor impact of post-glycosylation modifications
We also support chemical modifications such as:
N-Linked & O-Linked Glycosylation Pathway
Step-by-Step: N-Linked Glycosylation Pathway
The N-linked glycosylation pathway begins early during protein synthesis in the ER and continues with complex remodeling in the Golgi. Here's how we help decode each stage:
-
Synthesis of Lipid-Linked Oligosaccharides (LLOs)
Our platform detects dolichol-linked glycan intermediates with high sensitivity, ideal for early-pathway analysis.
-
Flipping and Transfer
We monitor flipping across the ER membrane and the action of the OST complex with N-glycosylation analysis services, helping to pinpoint bottlenecks or defects.
-
ER Trimming
Through precise enzyme activity profiling, we assess glucose/mannose removal steps and their role in folding checkpoints.
-
Golgi Remodeling
We provide high-resolution analysis of branched, hybrid, or complex N-glycans using mass spectrometry and HPLC platforms.
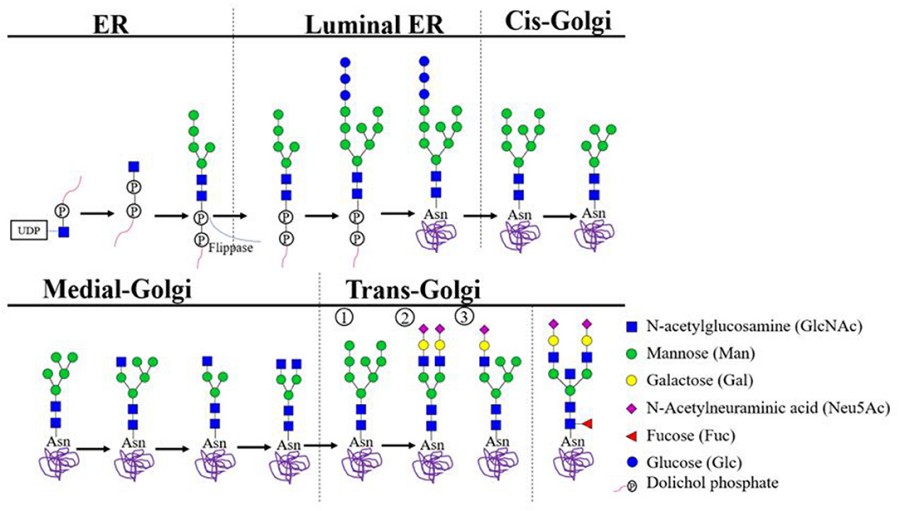 Fig.1 N-linked glycosylation pathway from ER to Golgi.1
Fig.1 N-linked glycosylation pathway from ER to Golgi.1
Every analysis is customizable—whether you need a single snapshot or full pathway tracking.
O-Linked Glycosylation: Beyond Mucins
O-glycosylation occurs later in the secretory pathway and is less predictable. But with our support, researchers can unravel even its most subtle variations. We integrate lectin microarrays with targeted MS approaches for comprehensive insight. To be more specific, our O-glycosylation analysis services help you:
-
Determine initiation by different GALNT enzymes
-
Map Core 1–8 structures with structural fidelity
-
Quantify mucin-type O-glycans in secreted proteins
-
Detect changes in adhesion or inflammatory markers
Comparing N-Linked with O-Linked Glycosylation
Understanding the differences between the two major types of protein glycosylation is key to selecting the right experimental strategy. Our platform is built to profile both with precision and scale. Whether you aim to compare glycoforms across species or investigate disease-associated glycan shifts, Creative Biolabs offers the tools and know-how to support your goals.
|
Feature
|
N-Linked Glycosylation
|
O-Linked Glycosylation
|
|
Initiation Site
|
Rough Endoplasmic Reticulum (ER)
|
Golgi Apparatus
|
|
Initial Sugar
|
N-acetylglucosamine (GlcNAc)
|
N-acetylgalactosamine (GalNAc)
|
|
Attachment Site
|
Asn in Asn-X-Ser/Thr motif
|
Ser/Thr without a strict motif
|
|
Assembly Pattern
|
Oligosaccharide added as a pre-assembled block
|
Sugars added one at a time
|
|
Timing
|
Co-translational
|
Post-translational
|
|
Functions
|
Folding, trafficking, immune shielding
|
Mucin function, adhesion, signal modulation
|
|
Our Support
|
N-glycosylation analysis for site-specific profiling
|
O-glycosylation analysis for glycoform mapping
|
Technology Spotlight: Our Most Powerful Tools
We combine these glycosylation analysis technologies under one roof—so you don't have to shop around for fragmented analysis.
Uncertain about the most suitable approach for your research? Don't hesitate to reach out to us for warm and professional consulting services! We are dedicated to providing you with customized solutions and professional guidance to help you overcome research challenges smoothly.
Extended Service Offerings
At Creative Biolabs, we know that glycosylation research doesn't end at structural analysis. That's why we provide additional specialized services to support every layer of your glycoprotein project:
Each of these services is powered by a dedicated team of glycobiologists, analytical chemists, and bioinformaticians—ready to help you tackle the complexity of glycosylation with clarity.
Glycosylation defines molecular identity. Whether you're mapping biosynthetic steps or modifying glycoproteins for function, you need precise tools and expert guidance. At Creative Biolabs, we deliver both. With advanced technologies, customizable workflows, and a deep understanding of glycan biology, we help you push glycosylation research further—from single-cell profiling to systems-level discovery. Contact us and start your glycosylation pathway project today!
References
-
Vletter, Esther M., et al. "A comparison of immunoglobulin variable region N-linked glycosylation in healthy donors, autoimmune disease and lymphoma." Frontiers in immunology 11 (2020): 241. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fimmu.2020.00241
-
He, Mengyuan, Xiangxiang Zhou, and Xin Wang. "Glycosylation: mechanisms, biological functions and clinical implications." Signal Transduction and Targeted Therapy 9.1 (2024): 194. https://doi.org/10.1038/s41392-024-01886-1
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 N-linked glycosylation pathway from ER to Golgi.1
Fig.1 N-linked glycosylation pathway from ER to Golgi.1

