Introduction
Glycosylation—the enzymatic addition of glycans to proteins or lipids—is a ubiquitous post-translational modification in eukaryotic systems. Viruses, particularly enveloped ones, hijack host-cell glycosylation machinery to decorate their surface glycoproteins, thereby influencing infectivity, host immune recognition, and vaccine design. Viral glycoproteins such as HIV-1 gp120 and SARS-CoV-2 spike proteins are heavily glycosylated, making glycomic profiling pivotal to virology, immunology, and therapeutic development.
Types of Glycosylation in Viruses
N-glycans are attached to asparagine residues within the consensus sequence N-X-S/T (X ≠ P). Viral N-glycans include:
-
High-mannose
-
Complex-type (sialylated and fucosylated)
-
Hybrid-type glycans
O-glycans are added to serine/threonine residues, primarily in mucin-type viruses. These include Core 1 (Galβ1-3GalNAc) and Core 2 structures, often sialylated.
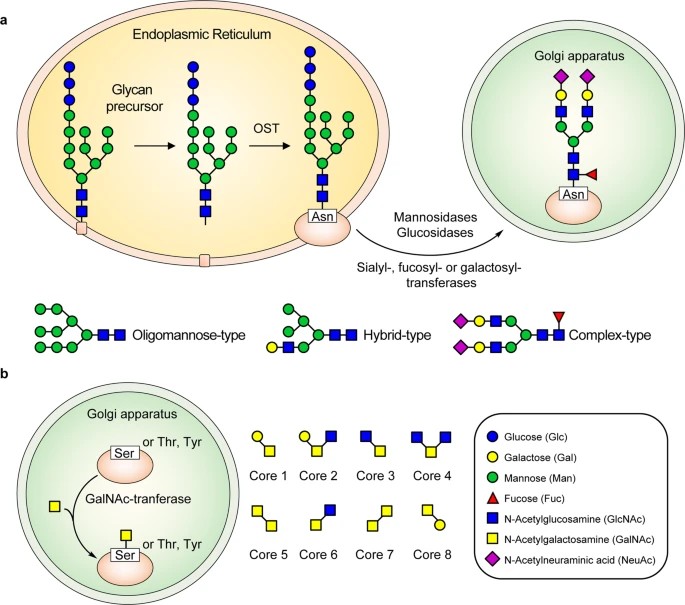 Fig.1 Schematic of N- and O-glycosylation pathways in SARS-CoV-2.1
Fig.1 Schematic of N- and O-glycosylation pathways in SARS-CoV-2.1
Viral Glycoproteins: Heavily Glycosylated Structures
|
Virus
|
Glycoprotein
|
Glycosylation Type
|
Function
|
|
HIV-1
|
gp120
|
N-glycosylation
|
Immune evasion, CD4 receptor binding
|
|
SARS-CoV-2
|
Spike (S)
|
N- and O-glycosylation
|
ACE2 binding, membrane fusion, immune evasion
|
|
Influenza A
|
Hemagglutinin
|
N-glycosylation
|
Receptor binding, antigenic drift
|
|
Herpesvirus
|
gB, gC
|
O-glycosylation
|
Cell entry, tropism
|
|
Hepatitis B
|
HBsAg
|
N-glycosylation
|
Immune modulation, vaccine target
|
|
Ebola virus
|
GP
|
N- and O-glycosylation
|
Host cell entry, immune modulation
|
Why Viruses Rely on Glycans
Glycosylation modulation enhances the immunogenicity of virus-like particles (VLPs):
-
Mannosylation of VLPs increases dendritic cell uptake.
-
Xylosylated RSV-F proteins produced in CHO cells showed improved immunogenicity.
-
Hyperglycosylated HBsAg VLPs accelerate B cell responses.
Viral envelope proteins, especially glycoproteins embedded in the virion membrane, are often heavily glycosylated. These glycan modifications are host-derived, and serve multifaceted roles:
-
Shielding neutralizing epitopes (e.g., HIV gp120 and SARS-CoV-2 spike protein)
-
Enhancing tropism and host receptor binding
-
Facilitating membrane fusion
-
Contributing to viral particle folding and stability
Virus-Specific Glycosylation Analysis Services
Creative Biolabs offers virus-specific glycosylation profiling services designed to support diverse research objectives—from decoding immune evasion mechanisms to guiding vaccine antigen design. Click on each virus type to view detailed service options.
|
Target Virus (Alphabetical)
|
What Can You Investigate?
|
How It Supports Your Research
|
|
COVID-19 (SARS-CoV-2)
|
-
Glycan shielding of spike protein
-
RBD glycosylation's role in ACE2 binding
-
Site-specific N-/O-glycan occupancy
|
-
Define immunogenic glycan sites
-
Inform vaccine design & bNAb targeting
|
|
Chikungunya Virus
|
-
Glycosylation on E1/E2 entry glycoproteins
-
Impact on cellular tropism
|
-
Investigate glycan-mediated entry
-
Support antiviral screening platforms
|
|
Dengue Virus
|
-
E protein glycosylation across serotypes
-
Study vector-host transmission influences
|
-
Identify conserved glycan features
-
Support serotype-specific vaccine development
|
|
Ebola Virus
|
-
Characterize glycan shield on GP1/GP2
-
Assess fucosylation/sialylation impact on virulence
|
-
Map epitope accessibility
-
Enhance therapeutic antibody efficacy
|
|
HIV
|
-
High-mannose clustering on gp120
-
Glycan-dependent epitope mapping
|
-
Guide broadly neutralizing antibody screening
-
Evaluate vaccine glycan mimicry
|
|
Hepatitis C Virus (HCV)
|
-
E1/E2 envelope glycan heterogeneity
-
Characterize glycosylation masking of epitopes
|
-
Reveal targets for neutralizing antibody design
-
Inform antiviral therapeutic strategies
|
|
Influenza Virus
|
-
HA/NA glycosylation site variation
-
Host adaptation and zoonotic shift mapping
|
-
Improve strain selection for vaccines
-
Analyze antigenic drift driven by glycosylation
|
|
MERS-CoV
|
-
Spike and envelope glycoprotein profiling
-
Identify immune evasion-related glycans
|
-
Understand receptor binding specificity
-
Support monoclonal antibody development
|
|
Measles Virus
|
-
F and H protein glycosylation profiling
-
Study effects on fusion and immune recognition
|
-
Aid vaccine optimization
-
Characterize immune escape pathways
|
|
Marburg Virus
|
-
Glycan structure-function relationships in GP
-
Host receptor interaction through glycan motifs
|
-
Discover novel intervention targets
-
Characterize immune response modulation
|
|
Multi-Virus Profiling
|
-
Cross-comparison of glycan motifs
-
Pan-virus glycan epitope identification
|
-
Enable broad-spectrum vaccine research
-
Benchmark interspecies glycosylation patterns
|
|
Rabies Virus
|
-
G protein glycan mapping across lineages
-
Trace evolution of epitope masking
|
-
Enhance next-gen vaccine constructs
-
Detect strain-specific glycan signatures
|
|
SARS-CoV
|
-
Compare glycan profiles with SARS-CoV-2
-
Characterize S protein glycosylation in zoonotic origins
|
-
Elucidate evolutionary glycan shifts
-
Assist in pan-coronavirus vaccine efforts
|
Glycoengineering Strategies and Analytical Support
At Creative Biolabs, we offer a suite of tailored services to decode viral glycosylation:
Glycoengineering Support: Custom glycan remodeling and host cell glycosylation tuning (CHO, HEK293, insect) to control product quality and immunogenicity.
N-Glycosylation Analysis: Precise identification and characterization of N-linked glycosylation sites and glycoforms using advanced LC-MS/MS.
O-Glycosylation Analysis: Profiling of O-linked glycosylation sites, especially in viral proteins, to understand immune shielding and receptor binding.
Fc Region Glycosylation Analysis: Detailed analysis of glycosylation patterns in the Fc region to optimize antibody functionality and stability.
Glycosylation Inhibition Assays: Screening of inhibitors targeting glycosylation pathways to assess their impact on viral infectivity and therapeutic efficacy.
High-Throughput Glycomic Analysis: Large-scale analysis of glycans from minimal sample inputs, providing in-depth glycan profiling for high-throughput applications.
Specialized Glycoconjugate Analysis: Analysis of complex glycoconjugates like glycoRNA and glycolipids, offering insights into their roles in disease and viral mechanisms.
Integrated Glycosylation Solutions: End-to-end solutions combining glycoengineering, DNA repair-linked glycan profiling, and glycoproteomic analytics for advanced research.
Glycosylation is not a passive decoration on viral proteins; it is a dynamic, functional determinant of viral infectivity, immune evasion, and host interactions. Dissecting viral glycoprotein not only deepens our understanding of pathogenesis but also reveals new avenues for vaccine and antiviral development. At Creative Biolabs, we provide cutting-edge solutions for viral glycoprotein analysis, including glycoprotein synthesis and analysis, glycoengineering, and immunogenicity assessment. Our robust analytical pipeline supports high-resolution viral glycomics, essential for immunogen optimization and therapeutic antibody validation. Contact us to accelerate your viral glycosylation research.
FAQs
Q: What is glycosylation in viruses?
A: Viral glycosylation is the process by which glycans are covalently attached to viral envelope proteins, such as HIV gp120 or the SARS-CoV-2 spike protein. This modification is mediated by the host's endoplasmic reticulum (ER) and Golgi apparatus, involving both N-linked and O-linked glycosylation pathways. Glycosylation influences protein folding, structural stability, and antigenicity, thereby impacting viral infectivity and the ability to evade the immune system. Characterizing these glycosylation patterns is essential for the development of vaccines and antiviral strategies, as they directly affect viral-host interactions. At Creative Biolabs, we provide comprehensive glycoprotein analysis services to map viral glycosylation profiles with site-level resolution, facilitating structure-function insights crucial for vaccine and antiviral development.
Q: What is the role of glycosylation in viral pathogenesis?
A: Glycosylation contributes to viral pathogenesis through multiple mechanisms: it masks immunogenic epitopes, modulates receptor binding affinity, and facilitates viral entry into host cells. For instance, in SARS-CoV-2, site-specific glycans at positions N165 and N234 stabilize the receptor-binding domain (RBD) of the spike protein, enhancing viral infectivity. Viruses often utilize host-like glycan structures to avoid immune recognition, highlighting the evolutionary adaptation of glycosylation in evading host defenses. Altering glycosylation profiles has been shown to reduce viral virulence, identifying glycosylation as a potential therapeutic target. Creative Biolabs supports glycosylation inhibition assays and site-directed glycan engineering to probe the functional consequences of glycosylation in viral pathogenesis.
Q: What does the glycoprotein do in a virus?
A: Viral glycoproteins are critical for viral attachment, membrane fusion, and entry into host cells, and they serve as primary antigens during infection. Examples include the SARS-CoV-2 spike protein and influenza hemagglutinin (HA), which are embedded in the viral envelope and undergo extensive glycosylation to evade immune detection. Glycan structures on these proteins also influence viral assembly and host cell tropism. Due to their role as major immune targets, viral glycoproteins are central to the design of vaccines and therapeutic antibodies. At Creative Biolabs, we specialize in glycoprotein research to support advancements in virus-related glycobiology.
Reference
-
Gong, Yanqiu, et al. "The glycosylation in SARS-CoV-2 and its receptor ACE2." Signal Transduction and Targeted Therapy 6.1 (2021): 396. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1038/s41392-021-00809-8
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Schematic of N- and O-glycosylation pathways in SARS-CoV-2.1
Fig.1 Schematic of N- and O-glycosylation pathways in SARS-CoV-2.1