Introduction to Receptor Glycosylation
Glycosylation is far from being a mere passive decoration; it represents a fundamental regulatory mechanism in receptor biology. As a pivotal post-translational modification, it directly governs membrane receptor folding, trafficking, and function. N- and O-linked glycans on receptors guide proper endoplasmic reticulum folding, prevent aggregation, and ensure structural stability. These moieties extend receptor half-life and fine-tune ligand accessibility and binding affinity. Beyond structure, glycosylation controls receptor localization, mediates endocytosis/recycling, and modulates clustering/dimerization for signaling. Glycans can mask immunogenic epitopes or create glyco-specific motifs, shaping immune recognition. Aberrant patterns link to diseases like cancer, infection, and immune disorders, making glycosylation profiling a powerful tool in receptor-targeted research.
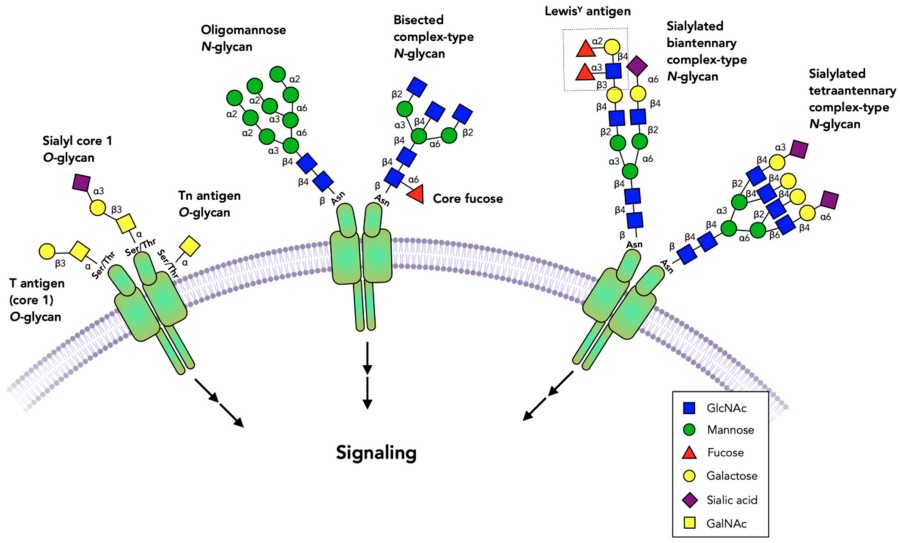 Fig.1 Glycosylated receptors in the extracellular domains.1
Fig.1 Glycosylated receptors in the extracellular domains.1
At Creative Biolabs, we help you to explore how receptor glycosylation drives molecular recognition and disease progression across a spectrum of critical receptor classes, including ACE2, GPCRs, EGFR, CFTR, PD-L1, and LAMP1. Our glycosylation analysis services enable high-resolution, site-specific glycan profiling across receptor systems to support your biomedical research.
N-Glycosylation for Structural Maturation
N-glycosylation is a co-translational modification in which oligosaccharide chains are covalently attached to asparagine residues within the consensus sequence Asn-X-Ser/Thr (where X ≠ Proline). This process occurs in the endoplasmic reticulum (ER) and is essential for the proper folding and maturation of receptor proteins. The initial attachment of a precursor glycan (Glc₃Man₉GlcNAc₂) is followed by glucose trimming and folding quality control, facilitated by calnexin and calreticulin. Misfolded or unglycosylated receptors are typically retained in the ER and targeted for degradation via the ER-associated degradation (ERAD) pathway.
Once correctly folded, receptors are exported to the Golgi, where the glycans undergo maturation into hybrid or complex forms. These complex-type N-glycans often carry terminal sialic acids, fucose, or bisecting GlcNAc structures, which fine-tune receptor function by influencing ligand affinity, receptor dimerization, and surface retention. For many membrane proteins, such as EGFR, GPCRs, and ACE2, N-glycosylation ensures structural integrity and modulates signal responsiveness and endocytic recycling.
O-Glycosylation in Receptor Regulation
O-glycosylation involves the enzymatic addition of monosaccharides—most commonly N-acetylgalactosamine (GalNAc)—to the hydroxyl groups of serine or threonine residues. This process initiates in the Golgi and proceeds without a strict sequence motif, making its prediction and analysis more complex than N-glycosylation. O-glycans are particularly enriched in extracellular, extended, or disordered regions of membrane proteins, including mucin-like domains.
This modification serves several critical functions. It enhances structural flexibility in extracellular loops, allowing dynamic movement that facilitates ligand interactions or conformational changes. O-glycans also act as a protective barrier against proteolytic cleavage, especially in immune checkpoint molecules and lysosomal membrane proteins. In immune modulation, O-glycosylation can create or conceal epitopes that interact with galectins, Siglecs, and antibodies, thereby altering immunogenicity. Receptors like PD-L1, LAMP1, and GPCRs leverage O-glycans for maintaining surface stability, avoiding degradation, and regulating downstream signaling.
|
Aspect
|
Impact of O-Glycosylation
|
|
Structural Flexibility
|
Increases conformational dynamics in extracellular loops
|
|
Protease Resistance
|
Shields cleavage sites from proteolytic enzymes
|
|
Immune Modulation
|
Modulates antigenicity and immune evasion
|
|
Mucin-domain Function
|
Essential for barrier proteins like LAMP1 and MUC1
|
|
Ligand Binding
|
Influences accessibility and spatial orientation of binding interfaces
|
Summary: N- vs O-Glycosylation in Receptors
|
Feature
|
N-Glycosylation
|
O-Glycosylation
|
|
Initiation Site
|
Endoplasmic Reticulum
|
Golgi apparatus
|
|
Consensus Sequence
|
Asn-X-Ser/Thr (X ≠ Pro)
|
None
|
|
Structural Role
|
Folding, stability
|
Flexibility, protease resistance
|
|
Key Receptor Examples
|
ACE2, EGFR, CFTR, PD-L1
|
PD-L1, LAMP1, MUC1, GPCR loops
|
|
Analytical Approach
|
Mass spectrometry, PNGase F mapping
|
ETD-MS, lectin arrays, mucin glycopeptide enrichment
|
A Case Example: Plant Cell-Surface Receptor Glycosylation
In plant systems, N-glycosylation plays a crucial role in the function of receptor-like kinases (RLKs) such as EFR and FLS2 in Arabidopsis. For EFR, proper glycosylation is required for ligand-induced receptor dimerization, while for FLS2, it is essential for recognizing bacterial flagellin and initiating immune responses. Mutations that disrupt glycosylation motifs impair receptor folding and functionality, underscoring the evolutionary conservation and structural necessity of this modification across species.
Specific Glycosylation in Receptor Function and Disease
Receptor glycosylation plays a fundamental role in modulating membrane protein behavior across diverse biological systems. From viral entry to immune regulation and cancer progression, both N- and O-linked glycosylation patterns shape receptor conformation, trafficking, stability, and ligand interactions. In particular, heavily glycosylated receptors such as ACE2, GPCRs, EGFR, CFTR, PD-L1, and LAMP1 serve as models for understanding how specific glycan structures dictate functional outcomes. At Creative Biolabs, we support researchers decoding the functional impact of receptor glycosylation—one of the most critical and structurally complex post-translational modifications in membrane biology. Whether you're investigating viral entry, immune evasion, or therapeutic resistance, our comprehensive glycosylation profiling services are designed to resolve site-specific glycosylation events and their biological consequences.
ACE2 Glycosylation and Viral Entry Mechanisms
The angiotensin-converting enzyme 2 (ACE2), known as the cellular entry point for SARS-CoV-2, carries at least seven conserved N-glycosylation sites. Among these, N53 and N90 are located directly adjacent to the spike protein-binding interface and critically shape viral binding affinity. Mutating or removing these glycans can either expose binding surfaces—enhancing viral entry—or destabilize ACE2, reducing infectivity. Meanwhile, glycosylation at distal sites like N322 and N546 stabilizes ACE2's global conformation and shields it from host proteases such as TMPRSS2. Additionally, glycoform heterogeneity—such as differential sialylation or core fucosylation—may underlie tissue-specific susceptibility to infection in the respiratory and cardiovascular systems.
|
N-Glycosylation Site
|
Functional Role
|
|
N53, N90
|
Directly impact SARS-CoV-2 S-protein binding
|
|
N322, N546
|
Modulate receptor stability and protease accessibility
|
To support such investigations, Creative Biolabs offers robust N-glycosylation analysis services that enable high-resolution, site-specific profiling of ACE2 glycan structures. Our platform allows researchers to correlate glycosylation with receptor stability, viral binding, and epitope accessibility—insights that are instrumental for rational vaccine design and therapeutic antibody development.
GPCR Glycosylation: Structural Maturation and Signaling Modulation
G protein-coupled receptors (GPCRs) are frequently modified by both N- and O-linked glycans, particularly in their extracellular N-terminal regions. These modifications are not decorative—they influence proper folding, plasma membrane trafficking, and ligand access. In addition, altered glycosylation patterns have been shown to affect G-protein coupling efficiency and signal desensitization dynamics. For instance, glycosylation of CCR5 modulates its function as a co-receptor for HIV-1. Creative Biolabs provides tailored glycan mapping and O-glycosylation analysis to support GPCR research. Our services span host cell systems, glycoform quantification, and receptor-ligand interaction studies, enabling comprehensive functional interpretation.
EGFR Glycosylation in Cancer Signaling
The epidermal growth factor receptor (EGFR) contains multiple N-glycosylation sites within its extracellular domain. These glycans are critical regulators of receptor behavior:
-
Certain glycans stabilize ligand-bound conformations, enhancing signaling specificity.
-
Hyperglycosylation promotes EGFR dimerization and persistent oncogenic activation.
-
In clinical contexts, glycan shielding may reduce accessibility of therapeutic monoclonal antibodies.
Through our N-glycosylation analysis services, Creative Biolabs enables detailed characterization of EGFR glycoforms, which is essential for drug response prediction and antibody-based therapeutic optimization.
CFTR Glycosylation and Folding Efficiency
The cystic fibrosis transmembrane conductance regulator (CFTR) contains two conserved N-glycosylation sites.
-
Proper glycosylation is required for apical localization in epithelial cells.
-
Misfolded glycoforms are retained in the ER and degraded.
-
Glycan trimming influences folding correctors in CF drug development.
Our N-glycosylation analysis services allow for the in-depth assessment of CFTR glycan processing and folding status, offering insights into therapeutic mechanisms and mutation-specific responses.
PD-L1 Glycosylation and Tumor Immune Escape
Programmed death-ligand 1 (PD-L1) is regulated by both N- and O-linked glycosylation. These modifications significantly affect its surface expression and immune function:
-
N-glycosylation at residues N192 and N200 stabilizes PD-L1 and prevents proteasomal degradation.
-
O-glycosylation near its extracellular domain influences immune recognition and protease resistance.
-
Hypo-glycosylation improves anti-PD-L1 antibody binding and enhances immunotherapeutic efficacy.
To investigate the therapeutic potential of glycan manipulation, Creative Biolabs provides glycosylation inhibition services. We offer inhibitor screening, functional glyco-modulation, and deglycosylation strategies to help researchers evaluate checkpoint blockade sensitization in cancer models.
LAMP1 Glycosylation and Lysosomal Function
Lysosome-associated membrane protein 1 (LAMP1) is among the most heavily glycosylated proteins, with extensive N- and O-glycan modifications that form a protective shield in the harsh lysosomal environment. These glycans:
-
Prevent degradation by lysosomal hydrolases.
-
Stabilize mucin-like extracellular domains.
-
Facilitate proper trafficking via the mannose-6-phosphate pathway.
Creative Biolabs supports lysosomal glycoprotein research through customized analysis workflows that characterize glycan architecture and function in sorting and membrane stability.
Integrated Glycosylation Analysis at Creative Biolabs
Creative Biolabs delivers end-to-end glycan profiling and functional analysis for glycosylated receptors across species and disease models. Our Featured Services Include:
Receptor glycosylation represents a functional code embedded within protein structure. Understanding and manipulating this code is vital for advancing receptor-targeted therapies and diagnostics. Whether it's ACE2-mediated viral entry, PD-L1-driven immune escape, or EGFR signaling in cancer, glycosylation dictates receptor biology from the ER to the immune synapse. Creative Biolabs provides advanced glycoscience tools and custom analysis services to illuminate the glycan landscape of membrane receptors and support innovation in drug discovery and biomarker development. Contact us and start your glycosylation analysis journey with Creative Biolabs today!
Reference
-
Gao, Yin, et al. "Role of glycans on key cell surface receptors that regulate cell proliferation and cell death." Cells 10.5 (2021): 1252. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/cells10051252
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Glycosylated receptors in the extracellular domains.1
Fig.1 Glycosylated receptors in the extracellular domains.1

