Creative Biolabs Empowering Precision in Inflammatory Bowel Disease (IBD) Research
Are you currently facing challenges in biomarker discovery or elucidating complex disease mechanisms in IBD? IBD, comprising Crohn's disease (CD) and ulcerative colitis (UC), represents a group of chronic inflammatory conditions of the gastrointestinal tract. Characterized by complex etiology and heterogeneous clinical presentations, IBD diagnosis, prognosis, and treatment response prediction remain significant challenges. Protein glycosylation is a fundamental post-translational modification that has profound effects on biological functions and immune regulation. Aberrant glycosylation is increasingly recognized as central to IBD pathogenesis, prediction, and response to therapy, offering novel diagnostic and therapeutic avenues to revolutionize patient care. Creative Biolabs' IBD-related glycosylation analysis service helps you accelerate IBD research, identify novel biomarkers, and elucidate disease pathogenesis through our advanced Glycoprotein Analysis platforms and high-resolution analytical techniques. Our service provides clear deliverables, solutions, and key problem-solving capabilities to advance your IBD research. We specialize in uncovering the subtle yet profound roles of glycans in disease initiation, progression, and therapeutic response, moving beyond traditional markers to unlock new dimensions of biological understanding. Our insights empower you to make more informed decisions, from early disease prediction and non-invasive diagnostics to identifying novel therapeutic targets and understanding complex disease mechanisms.
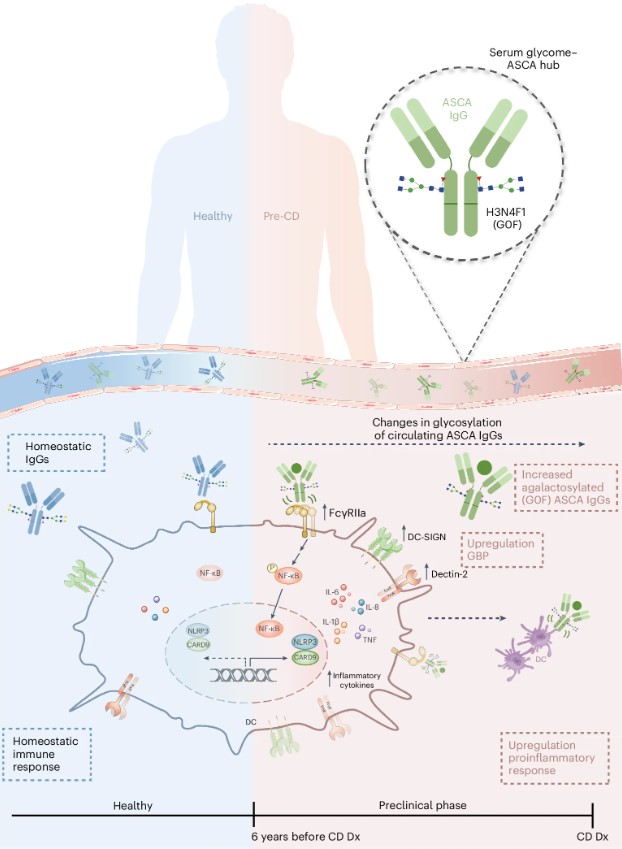 Fig.1 Altered IgG Fc glycosylation in CD disease.1,3
Fig.1 Altered IgG Fc glycosylation in CD disease.1,3
Process of Our IBD related Glycosylation Analysis Service
Creative Biolabs employs a highly integrated, multi-platform approach to deliver accurate and comprehensive Glycosylation Analysis Services.
-
Standardized Sample Preparation
We utilize proprietary and optimized protocols for extracting and preparing glycans from diverse biological matrices. This includes specific enzymatic treatments (e.g., PNGase F for N-glycans) and chemical methods (e.g., reductive β-elimination for O-glycans) to cleanly release glycans from proteins. Subsequent steps involve fluorescent labeling (e.g., with per-methylation for MS) to enhance detection sensitivity, followed by meticulous purification steps to remove interfering substances.
-
Advanced Analytical Platforms
Our state-of-the-art laboratory houses a comprehensive suite of high-resolution instruments:
-
Ultra-High Performance Liquid Chromatography (UHPLC) / High Performance Liquid Chromatography (HPLC): The gold standard for high-throughput separation and quantification of fluorescently labeled N-glycans. This allows for precise measurement of relative abundances of individual glycan peaks and the calculation of derived glycan traits.
-
Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF MS): The powerful mass spectrometry platform provides unparalleled sensitivity and detailed structural information. They are critical for the identification of novel glycoforms, confirmation of known structures, and quantitative analysis of both N-linked and O-linked glycans.
-
Capillary Electrophoresis (CE): Offering exceptional resolution and high-throughput capabilities, CE allows for rapid and automated N-glycan profiling.
-
Immunoblotting & Immunohistochemistry: Used for the detection and localization of specific glycosylated proteins (e.g., O-GlcNAc levels in tissue sections or cell lysates) and glycan-binding proteins, providing complementary qualitative and semi-quantitative data.
-
Flow Cytometry: Essential for functional assays and for analyzing glycosylation patterns directly on the surface of isolated immune cells.
-
Robust Bioinformatic Processing
Raw data from our analytical platforms undergoes rigorous processing using advanced bioinformatics pipelines. This involves automated peak integration, alignment, and normalization. Our team then applies sophisticated statistical methodologies, including multivariate analysis and logistic regression, to identify significant glycan changes.
-
Expert Biological Interpretation
Beyond data generation, Creative Biolabs excels in providing deep biological interpretation. Our experienced glyco-biologists translate complex glycomics data into meaningful insights, elucidating mechanistic roles, identifying potential biomarkers, and suggesting avenues for therapeutic intervention. We provide comprehensive reports, including graphical representations and detailed explanations, ensuring that the results are clear, actionable, and directly address your research questions.
Key Advantages
-
Unrivaled Expertise: Our team comprises seasoned glyco-biologists and analytical chemists with decades of experience in deciphering complex glycan structures and their biological significance in inflammatory diseases.
-
State-of-the-Art Platforms: We utilize an advanced suite of analytical technologies to ensure comprehensive and high-resolution glycolprofiling.
-
Comprehensive Glycan Analysis: We offer end-to-end solutions for both N-linked and O-linked glycosylation, covering diverse sample types from serum and tissue to non-invasive fecal samples, providing a holistic view of glycosylation changes.
-
Predictive & Prognostic Power: Our services enable the discovery and validation of highly sensitive and specific glycan biomarkers for early disease prediction and monitoring therapeutic efficacy.
-
Mechanistic Insights: We go beyond data generation, providing in-depth biological interpretations that uncover the functional roles of glycan alterations in disease pathogenesis and their impact on immune responses, cellular signaling, and mucosal barrier integrity.
-
Economic Efficiency: Our optimized workflows and cutting-edge technology lead to efficient project execution and provide valuable insights.
Creative Biolabs is dedicated to advancing your IBD research with unparalleled expertise and cutting-edge technologies. Our IBD related glycosylation analysis service provides critical insights into disease mechanisms, predictive biomarkers, and novel therapeutic avenues, empowering you to make significant breakthroughs in personalized medicine. Our team of expert biology specialists is eager to discuss your specific project needs and provide tailored solutions. Please contact us to inquire about more details.
Published Data
This research investigates the shifts in immunoglobulin G (IgG) N-glycan structures among individuals with CD who are undergoing anti-TNF biological treatments. The central finding indicates that these therapies lead to beneficial alterations in the IgG N-glycome. Over a 14-week treatment period, there was a noticeable reduction in IgG agalactosylation, accompanied by an increase in forms with monogalactosylation, digalactosylation, and sialylated galactose residues. These changes collectively point towards a reduction in the inflammatory state characteristic of CD, suggesting that anti-TNF drugs effectively modulate the immune system in a way that alleviates inflammation.
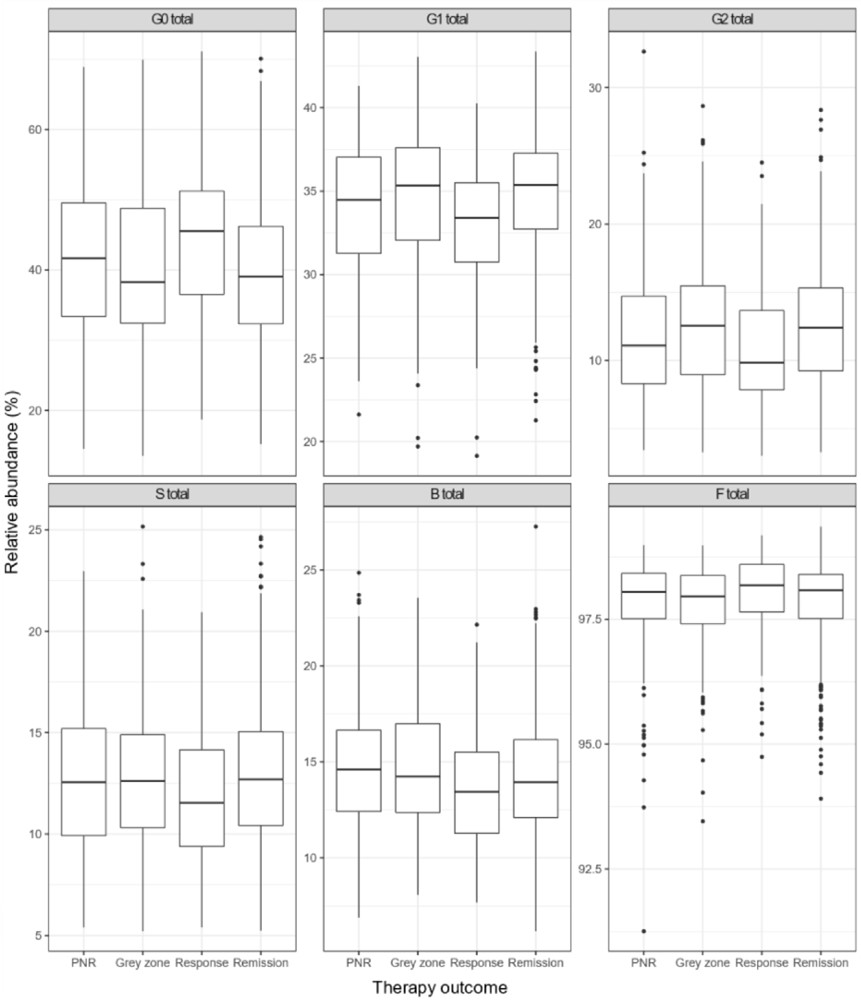 Fig.2 Relative abundance of IgG N-glycan features between groups.2,3
Fig.2 Relative abundance of IgG N-glycan features between groups.2,3
FAQs
Q1: How can Creative Biolabs' glycomics services help differentiate IBD from Irritable Bowel Syndrome (IBS)?
A1: Our fecal mucin glycosylation analysis service is specifically designed for this purpose. We've identified unique O-glycan signatures in fecal samples from Crohn's disease patients (e.g., decreased sialylated glycans and increased truncated core 1 O-glycans) that are distinct from those in IBS patients or healthy individuals. This non-invasive approach provides a powerful tool for differential diagnosis, potentially reducing the need for invasive endoscopy. Contact us to learn more about our non-invasive analysis capabilities!
Q2: What types of samples do you accept for IBD glycosylation analysis, and how should they be prepared?
A2: We accept a wide range of biological samples, including serum, plasma, fecal samples, and intestinal tissue biopsies. For optimal results, we provide detailed sample collection and storage guidelines upon project initiation. Our team will guide you through the appropriate preparation steps to ensure sample integrity and maximize the success of your glycomics analysis.
Q3: What is the typical turnaround time for an IBD glycosylation analysis project, and what factors influence it?
A3: The typical timeframe for a comprehensive IBD glycosylation analysis project ranges from 8 to 12 weeks. Key factors influencing the duration include the volume of samples, the complexity of the glycan types targeted (e.g., N-linked, O-linked, specific glycoforms), and the depth of bioinformatic and biological interpretation required. We prioritize both speed and accuracy. For a tailored estimate, please provide details about your project scope.
Customer Review
Unlocking Early Diagnosis
"Using Creative Biolabs' IBD glycosylation analysis service has significantly improved our ability to identify early-stage Crohn's disease patients, providing crucial diagnostic clarity that endoscopy often misses in its initial stages. The accuracy is unparalleled." - Dr. D. Gra***m.
Non-Invasive Diagnostic Leap
"The fecal mucin glycosylation analysis offered by Creative Biolabs is a game-changer. It's a truly non-invasive method that has allowed us to differentiate IBD from IBS with remarkable specificity, reducing the need for repeated invasive procedures and significantly improving patient comfort. The detailed glycan signatures are invaluable." - Prof. F. Irr***s.
References
-
Gaifem, Joana, et al. "A unique serum IgG glycosylation signature predicts development of Crohn's disease and is associated with pathogenic antibodies to mannose glycan." Nature Immunology 25.9 (2024): 1692-1703. DOI: 10.1038/s41590-024-01916-8.
-
Hanić, Maja, et al. "Anti-TNF biologicals enhance the anti-inflammatory properties of IgG N-glycome in Crohn's disease." Biomolecules 13.6 (2023): 954. DOI: 10.3390/biom13060954.
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Altered IgG Fc glycosylation in CD disease.1,3
Fig.1 Altered IgG Fc glycosylation in CD disease.1,3
 Fig.2 Relative abundance of IgG N-glycan features between groups.2,3
Fig.2 Relative abundance of IgG N-glycan features between groups.2,3

