At Creative Biolabs, we specialize in making sense of protein glycosylation. As a leader in this field, we know that glycosylation is a major player in determining their function, stability, and how they interact with other molecules. We are here to help you understand why mapping glycosylation sites matters. We hold the firm belief that our services can help your research or project. There is a suite of services we provide, including but not limited to:
-
Glycosylation Site Mapping: Uncover N- and O-linked sites with sub-ppm mass accuracy, including quantification of site occupancy and profiling of glycoform diversity.
-
Virus Glyoprotein Analysis: Custom workflows to handle viral sample complexity, integrating glycan structure elucidation, functional annotation, and immune interaction analysis.
-
Custom Solutions: Adaptable to diverse sample types (proteins, cells, tissues, viruses) and project scales, from exploratory research to reliable quality control.
Why Mapping Glycosylation Sites Matters?
Glycosylation adds sugar chains to proteins, but where these chains attach (the "sites") is crucial. One protein can have multiple sites, each with different sugar structures. This variety means the same protein can act differently based on its glycosylation sites. For example, a sugar at one site might help a protein fold correctly, while a sugar at another site could let it bind to a virus or immune cell. In diseases, changes at specific sites can signal trouble, making them potential targets for new drugs or biomarkers. Our glycosylation site mapping services zero in on these sites, giving you detailed info about where sugars attach and what they do.
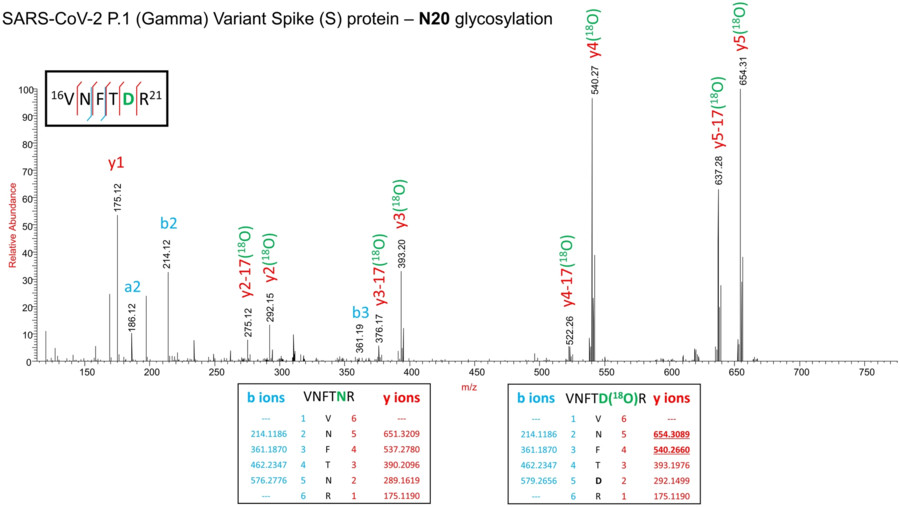 Fig.1 Specific glycosylation confirms the N-glycosylation at N20 site on gamma variant spike.1,3
Fig.1 Specific glycosylation confirms the N-glycosylation at N20 site on gamma variant spike.1,3
Two Types of Glycosylation: N-Linked and O-Linked
Proteins get sugars in two main ways, each with its own rules and challenges:
N-Linked Glycosylation: The "Asn" Spot
N-linked glycosylation requires for a specific amino acid sequence motif, known as the N-linked glycosylation sequon. These sugars attach to a specific amino acid called asparagine (Asn), but only if it's part of a certain sequence: Asn-X-Ser/Thr (where X is any amino acid except proline). Not every Asn in this sequence gets a sugar, though—factors like protein shape and cell environment matter. We use an enzyme called PNGase F to remove these sugars, leaving a tiny mass change that our mass spectrometers can detect. This helps us confirm which Asn sites are actually "occupied" by sugars.
O-Linked Glycosylation: The Ser/Thr Site
Here, sugars attach to serine (Ser) or threonine (Thr) amino acids. Unlike N-linked, there's no strict sequence rule, so predicting sites is harder. O-sugars can be simple or complex, and they're involved in key processes like cell signaling. We use a mix of chemical tricks, special enzymes (like OpeRATOR), and advanced mass spec to find these sites, even when they're hard to detect.
How We Map Sites: Our Step-by-Step Process
Our method is built on sensitivity and accuracy, using tools that handle even tricky samples:
1. Prepping Your Sample
Initially we extract the proteins you need from cells, tissues or fluids. During the sample preparation process we work to eliminate noise elements to concentrate on the important glycoproteins.
2. Breaking Proteins into Peptides
Our method requires enzymes such as trypsin to break down proteins into smaller peptide fragments. Some resulting peptides will contain attached sugars which makes them glycopeptides and these represent our primary targets.
3. Catching the Glycopeptides
Glycopeptides are often rare, so we use techniques to pull them out:
-
HILIC: Captures glycopeptides because their sugars are water-loving (hydrophilic).
-
Lectins: Proteins that stick to specific sugars, letting us fish out glycopeptides with those structures.
-
Chemical Tags: Adding labels that bind to sugar groups for easy isolation.
4. Mass Spectrometry: The Detective Work
Our mass spectrometers (MS) are like molecular detectives. They measure the mass of glycopeptides and break them apart (using methods like CID or ETD) to see both the peptide sequence and the sugar structure. For N-linked sites, we look for the mass change from Asn to Asp. For O-linked, we rely on detailed fragmentation patterns to pinpoint where the sugar is attached.
5. Making Sense of the Data
We use specialized software to match the MS data to known proteins, identify potential sites, and confirm which ones are really glycosylated. This step turns raw data into clear, usable insights.
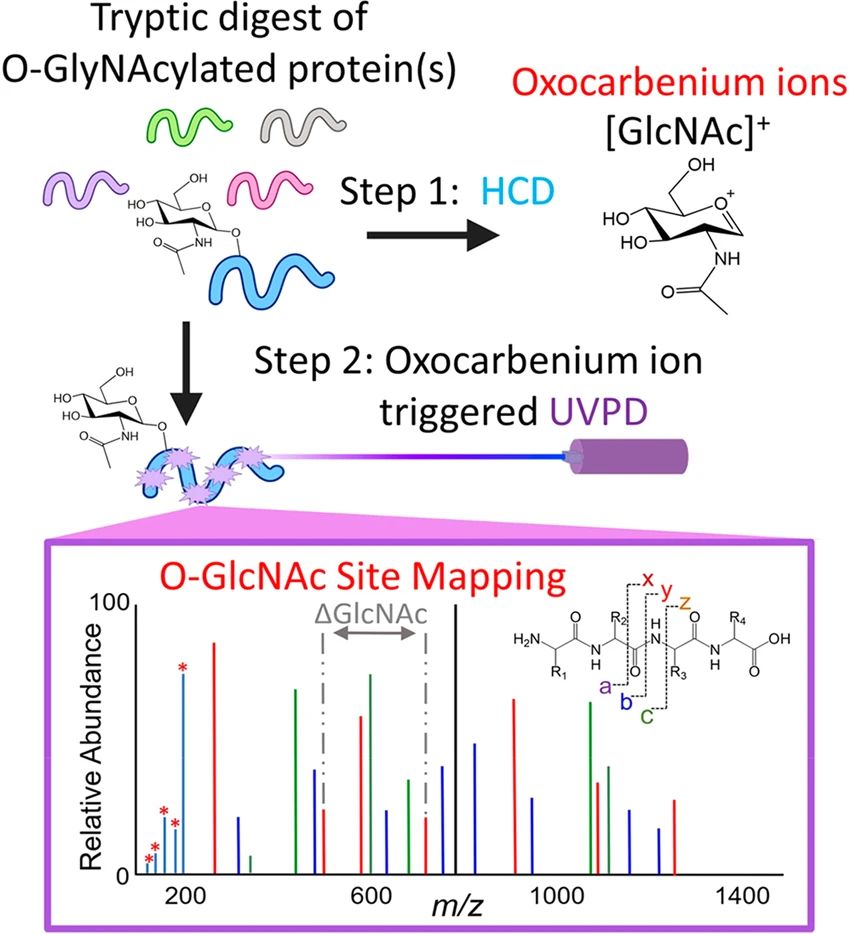 Fig.2 O-GlcNAc Site Mapping Using HCD and UVPD.2,3
Fig.2 O-GlcNAc Site Mapping Using HCD and UVPD.2,3
Why Choose Us for Glycosylation Analysis?
Our services stand out for a few key reasons:
-
Full Coverage: We handle both N-linked and O-linked sites, telling you exactly which amino acids have sugars and what those sugars look like.
-
Sensitive Tech: Our advanced MS machines (like Orbitrap and Q-TOF) can detect even tiny amounts of glycopeptides, perfect for rare or low-abundance samples.
-
Custom Solutions: Whether you're working on a cancer protein, a virus, or a therapeutic antibody, we tailor our methods to your needs.
If you're studying virus glycoproteins, our glycosylation analysis services for virus glycoprotein are a great fit. Viral sugars are key to how they infect cells and hide from the immune system. Our analysis can help you understand these sites, which is critical for developing vaccines or antiviral drugs.
How Our Services Help?
Our services make a difference in three key areas:
Drug Development
For biotech companies, we help ensure that therapeutic proteins—like antibodies—have the right glycosylation. This means making sure their sugar patterns are correct for safety and effectiveness. Accurate glycosylation data is essential for meeting regulatory standards and developing reliable treatments.
Disease Research
When studying diseases, we look for abnormal glycosylation in proteins linked to illness. By finding these changes, we can identify new biomarkers—these are like biological clues that signal a disease is present. Such clues can lead to earlier diagnosis or the development of targeted therapies that address the root cause.
Basic Science
In basic research, we aim to understand how sugars attached at specific sites affect how proteins work. This helps us decode fundamental cell processes, such as how the immune system responds to threats or how cells grow and communicate. These insights lay the groundwork for broader discoveries in biology.
We know this field isn't easy. Sugars come in many forms, some sites are only partly occupied, and samples can be messy. Here's how we deal with that:
-
Better Methods: We're always refining our enrichment and MS techniques to catch even the trickiest glycopeptides.
-
Smart Software: Our bioinformatics tools are designed to handle complex data, ensuring we don't miss subtle site signals.
-
Collaboration: We work closely with you to design experiments that fit your sample's unique needs—no one-size-fits-all approaches.
Whether you're a researcher exploring basic biology or a company developing the next big therapy, knowing where and how sugars attach to proteins is key. Our glycosylation site mapping services and glycosylation analysis services for virus glycoprotein are designed to give you clear, actionable data. At Creative Biolabs, we're here to make glycosylation analysis straightforward and effective. Contact us today to talk about how we can help your project—no jargon, just practical solutions. Let's turn complex glycans into clear insights, together.
References
-
Bissette, Andrew J. "Mapping protein glycosylation through oxocarbenium ion generation." Communications Chemistry 3.1 (2020): 115. https://doi.org/10.1038/s42004-020-00352-7
-
Shajahan, Asif, et al. "Site specific N-and O-glycosylation mapping of the spike proteins of SARS-CoV-2 variants of concern." Scientific Reports 13.1 (2023): 10053. https://doi.org/10.1038/s41598-023-33088-0
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Specific glycosylation confirms the N-glycosylation at N20 site on gamma variant spike.1,3
Fig.1 Specific glycosylation confirms the N-glycosylation at N20 site on gamma variant spike.1,3
 Fig.2 O-GlcNAc Site Mapping Using HCD and UVPD.2,3
Fig.2 O-GlcNAc Site Mapping Using HCD and UVPD.2,3

