Glycosylation is a common process. The process attaches sugar molecules called glycans to proteins along with lipids and multiple other biomolecules. This is not just a simple change. Glycosylation modifies the function of molecules while determining their cellular location and stability. Since glycosylation plays a critical role in therapeutic proteins and vaccines precise regulation of glycan composition becomes essential. The demand to control glycosylation pathways has led to glycoengineering development which modifies these pathways in host expression systems. Glycoproteins with specific glycan profiles matching human standards can be produced when researchers modify the glycosylation pathway enzymes in host cell lines like mammalian cells, insect, yeast, plants, or bacteria. Creative Biolabs possesses multiple years of expertise in glycosylation research. Different species exhibit their own distinct glycosylation patterns. Our expert team utilizes specialized tools to assist clients in mastering glycosylation patterns during research and development stages.
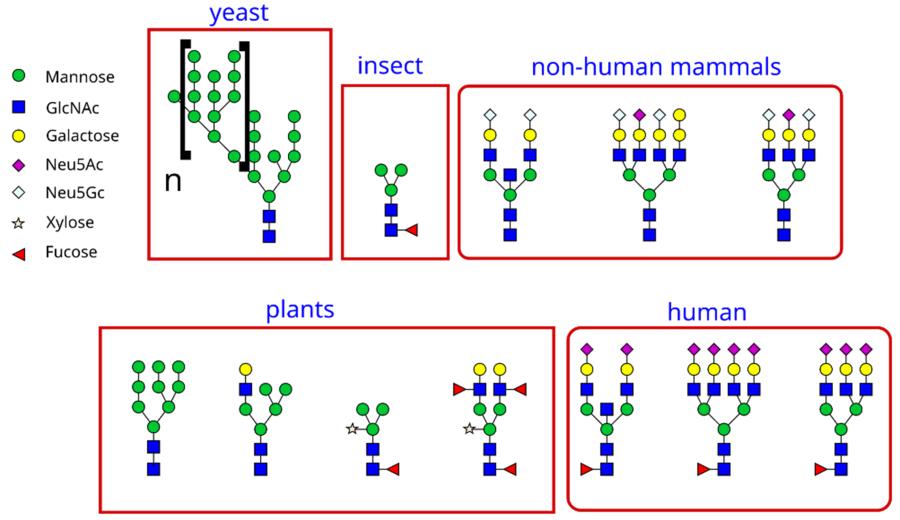 Fig.1 The different types of glycans in different organisms.Distributed under CC BY-SA 3.0, from Wiki, without modification.
Fig.1 The different types of glycans in different organisms.Distributed under CC BY-SA 3.0, from Wiki, without modification.
Two Main Types of Protein Glycosylation
There are two major ways proteins get glycosylated: N-linked and O-linked. While the basic ideas are similar in many organisms, the details differ between species.
N-linked Glycosylation
-
Happens when a sugar chain attaches to a specific amino acid called asparagine (Asn).
-
This only happens in a certain sequence: Asn-X-Ser or Asn-X-Thr (where X is any amino acid except proline).
-
Starts in the endoplasmic reticulum (ER) of cells. A large sugar precursor is transferred to the protein, then processed in the ER and Golgi to form mature sugars.
O-linked Glycosylation
-
Attaches sugar chains to serine (Ser) or threonine (Thr) amino acids.
-
More varied than N-linked. Sugars are added one by one in different parts of the cell, without a large precursor like in N-linked glycosylation.
-
A common example is mucin-type glycosylation, which starts with adding a specific sugar (GalNAc) to Ser/Thr.
Human Glycosylation
Human glycosylation makes many different sugar structures. These affect health and disease in several ways.
N-linked Glycosylation in Humans
-
Starts with a precursor sugar, then forms three types: high-mannose, hybrid, and complex glycans.
-
Complex glycans have outer chains with sugars like galactose, sialic acid, and fucose. The exact structure depends on the cell's enzymes.
O-linked Glycosylation in Humans
-
Often starts with GalNAc on Ser/Thr (mucin-type).
-
Shorter than N-glycans but important for cell signaling, cell adhesion, and protecting proteins from breakdown.
Why It Matters for Humans
-
Glycans help immune cells recognize pathogens. Changes in glycosylation are linked to diseases like cancer and inflammation.
-
Glycoproteins on cell surfaces send signals. Sugars can adjust how these signals work.
-
Helps proteins fold correctly and move to the right place in the cell.
Bacterial Glycosylation
Bacteria also use glycosylation, which helps them survive and cause disease.
N-linked Glycosylation in Bacteria
-
Less common than O-linked, found in some species like Campylobacter jejuni.
-
Uses different enzymes and sugar structures than in humans but has a similar process of transferring sugars to proteins.
O-linked Glycosylation in Bacteria
-
More common, attaching sugars to Ser/Thr.
-
Important for functions like flagella movement (motility), sticking to host cells (adhesion), and avoiding the immune system.
Glycosylation in E. coli
-
Pathogenic Strains vs. Lab Strains: Most laboratory E. coli strains do not glycosylate proteins, but pathogenic strains like enteroaggregative E. coli (EAEC) have functional systems.
-
TibA Adhesin Case: The TibA protein (translocated intimin-like protein A) in EAEC is heavily O-glycosylated with heptose sugars. This glycosylation is critical for TibA's stability and its role in bacterial clumping and sticking to human intestinal cells, helping EAEC cause infections.
Plant Glycosylation
Plants have their own glycosylation features, different from animals.
N-linked Glycosylation in Plants
-
Starts like in mammals but adds plant-specific sugars (xylose, fucose) that can trigger immune responses in humans.
O-linked Glycosylation in Plants
-
Includes proteins with long sugar chains (arabinogalactans), important for plant growth, development, and stress responses.
Distinctive Features of Plant Glycosylation
|
Feature
|
Description
|
Significance
|
|
β(1,2)-Xylose
|
Present in complex N-glycans, linked to core mannose.
|
Plant-specific epitope, potentially immunogenic in mammals.
|
|
α(1,3)-Fucose
|
Present in complex N-glycans, linked to core GlcNAc.
|
Plant-specific epitope, potentially immunogenic in mammals.
|
|
Arabinogalactans
|
Extensive, branched glycans linked to hydroxyproline in AGPs.
|
Crucial for AGP function in plant growth, development, and signaling.
|
|
Limited Sialylation
|
Sialic acid is generally absent in plant glycans.
|
Contrasts with the prevalence of sialic acid in mammalian glycans.
|
Viral Glycosylation
Viruses rely on host cell machinery for glycosylation. Glycans on viral proteins help them infect cells and avoid the immune system.
-
Glycan Shield: Sugars on viral envelope proteins (like those in COVID or flu viruses) hide the virus from antibodies.
-
Host Entry: Glycans help viruses attach to host cell receptors, starting the infection process.
-
Protein Folding and Assembly: Glycosylation is often essential for the proper folding, trafficking, and assembly of viral glycoproteins in the host cell's ER and Golgi.
-
Viral Budding and Release: Glycoproteins are integral to the process of viral budding from the host cell membrane.
Our glycosylation analysis services for virus glycoprotein can identify these sugar structures, aiding vaccine design and antiviral research. We provide comprehensive glycosylation analysis solutions spanning from common virus Influenza to hotspot counterpart like COVID-19:
Why Species Differences Matter
-
When making therapeutic proteins in cells (like CHO cells or bacteria), controlling glycosylation is key for safety and effectiveness. Our glycosylation site mapping services find where sugars attach, helping optimize protein design.
-
Differences in glycosylation between species (like humans and mice) mean research results may not always match. Researchers need to understand these differences for accurate disease modeling.
-
Changes in glycosylation can be markers for diseases like cancer, guiding new diagnostic tools.
A Case Example: Human vs. Mouse Vitronectin Glycosylation
As a glycoprotein present in both blood and tissues, Vitronectin (VTN) serves important functions in cell adhesion processes along with blood clotting mechanisms and immune system regulation. Human and mouse vitronectin share 82% amino acid similarity yet their glycosylation patterns show significant differences which demonstrate species-specific variations influencing protein function.
|
Human Vitronectin Glycosylation
|
-
Contains both N-linked (on asparagine, Asn) and O-linked (on serine/threonine, Ser/Thr) sites.
-
Key N-glycosylation sites are in the sequence Asn-X-Ser/Thr (X = any amino acid except proline).
-
Attached glycans are mostly "complex-type," with sialic acid and fucose, which influence VTN's shape, stability, and binding to molecules like integrins (cell adhesion proteins) and PAI-1 (a clotting regulator).
|
|
Mouse Vitronectin Glycosylation
|
-
Despite high sequence homology, amino acid differences alter potential glycosylation sites:
-
Some human N-X-S/T sites are missing in mice due to substitutions (e.g., Asn → Asp).
-
Mice may have unique glycosylation sites not present in humans.
-
Even conserved sites carry different glycans: Mouse cells may add fewer sialic acids or more core fucose, changing how VTN interacts with other molecules.
|
It Matters for Research. When studying human diseases using mice, mouse VTN's different glycosylation can lead to misleading results. For example, a drug targeting human VTN's sialic acid may not work in mice if their VTN lacks this modification. Researchers developing VTN-targeted therapies (e.g., for cancer or fibrosis) need to understand these differences to avoid off-target effects or reduced efficacy.
Creative Biolabs Services
We offer specialized services to address these needs:
-
Glycosylation Site Mapping: Identify exactly where sugars attach to proteins, crucial for understanding protein function and improving biopharmaceuticals.
-
Virus Glycoprotein Analysis: Study how glycosylation affects viral infection and immune evasion, supporting vaccine and drug development.
-
Glycoengineering: Modify cell systems to produce proteins with desired sugar patterns, reducing immune risks and improving efficacy.
Species-specific glycosylation is a key biological feature. Species-specific glycosylation impacts protein function within humans as well as bacteria, plants, and viruses which leads to significant effects on health, disease management, and scientific exploration. Creative Biolabs transforms complex glycosylation studies into straightforward processes. Our skilled team combined with advanced technology enables clients to understand species variations while developing improved therapies and discovering fresh scientific knowledge. We provide assistance for both protein sugar site mapping and viral glycan analysis. Reach out to us to find out more about our virus glycoprotein-focused glycosylation site mapping services and glycosylation analysis services.
References
-
Larsen, Joachim Steen, et al. "Engineering mammalian cells to produce plant-specific N-glycosylation on proteins." Glycobiology 30.8 (2020): 528-538. https://doi.org/10.1093/glycob/cwaa009
-
Sano, Kotone, et al. "Changes in glycosylation of vitronectin modulate multimerization and collagen binding during liver regeneration." Glycobiology 17.7 (2007): 784-794. https://doi.org/10.1093/glycob/cwm031
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 The different types of glycans in different organisms.
Fig.1 The different types of glycans in different organisms.
