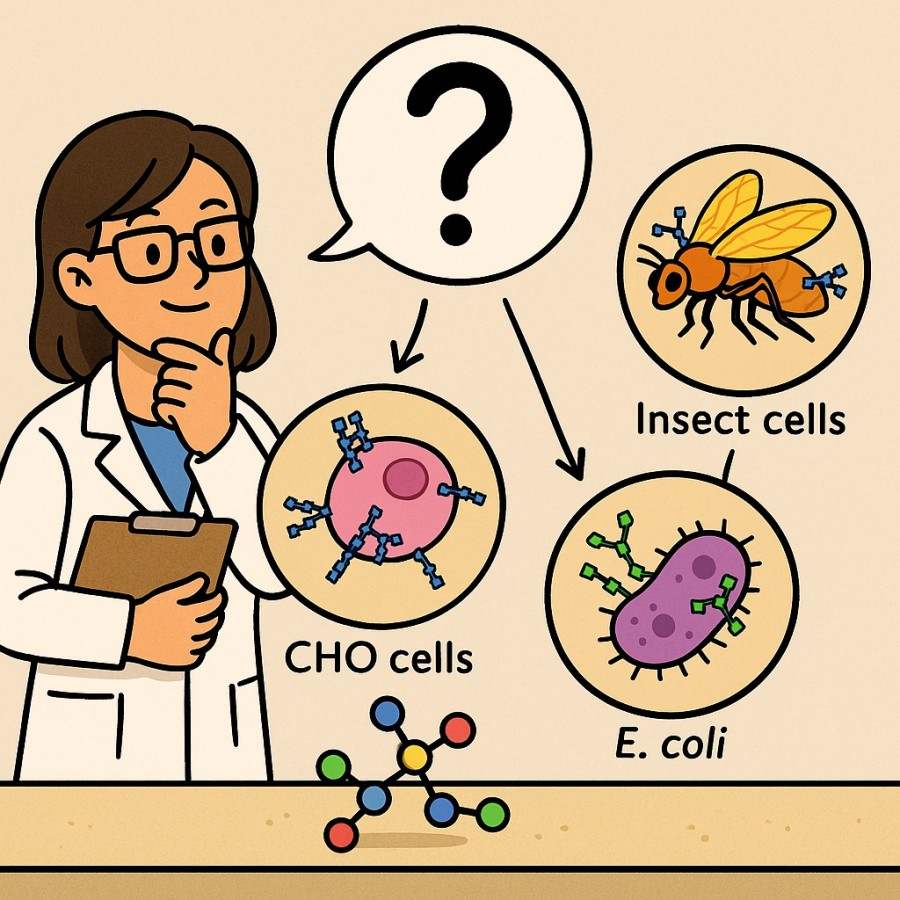
Why Use Different Glycosylation Expression Systems?
No single expression system can meet all glycosylation needs. Thus, you may need different glycosylation expression systems for different research goals. As protein glycosylation directly affects stability, half-life, folding efficiency, and immune recognition, choosing the optimal host is a critical early decision in any recombinant protein project. Each system—whether mammalian, insect, yeast, or bacterial—brings unique biosynthetic capabilities that can either enhance or hinder your intended application.
The optimal system depends on the biological function of your protein and the role of glycosylation within it. For example:
-
If you require precise control over sialylation or core fucosylation, mammalian systems like CHO are essential.
-
If you're focused on cost-efficient mass production of vaccine antigens or enzymes, insect cells or Pichia pastoris may offer a better yield-to-cost ratio.
-
If your target is a non-glycosylated protein or you're engineering custom glycan motifs, E. coli becomes a highly adaptable and scalable platform.
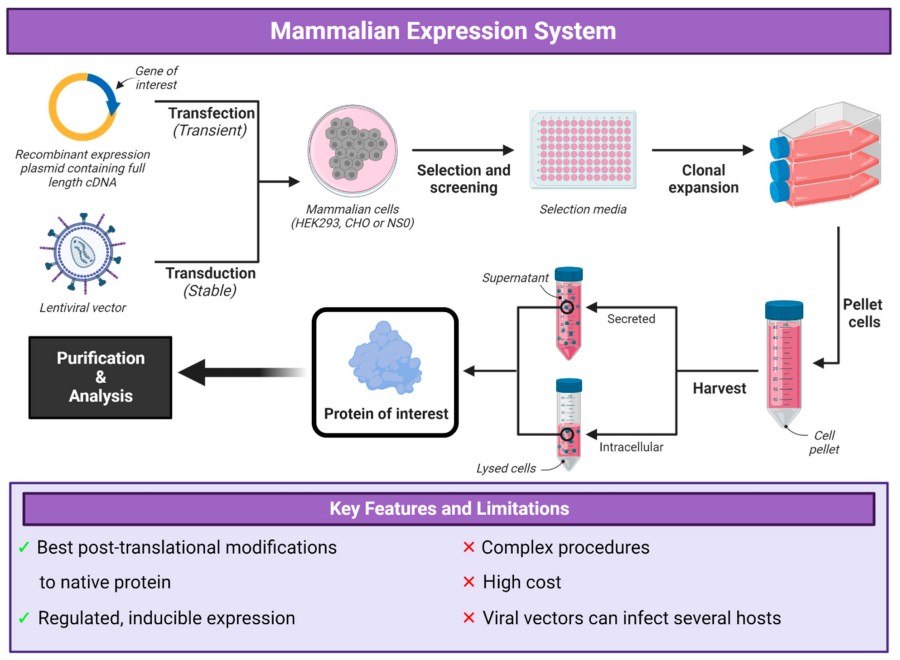 Fig.1 The workflow of mammalian expression system.1
Fig.1 The workflow of mammalian expression system.1
At Creative Biolabs, we support researchers in navigating this critical selection process. With decades of experience in glycosylation analysis and expression system engineering, we help match your protein's structural and functional needs to the right host system
How to Choose a Glycosylation Expression System?
Different host systems introduce distinct glycosylation patterns, which can significantly influence immunogenicity, receptor interactions, and therapeutic efficacy. Therefore, selecting the right system is not simply a technical detail, but a strategic decision based on your downstream objectives. The decision-making process typically involves:
-
Keeping your research goal in mind
-
Define the desired glycosylation type based on the protein's biological function and glycan dependence. (e.g., human-like, high-mannose, or none)
-
Evaluating production scale, cost, timeline, and regulatory acceptance, especially for translational applications.
To make an informed choice, start by clarifying your research purpose:
-
Are you optimizing Fc glycoforms for a monoclonal antibody?
-
Producing a non-glycosylated enzyme for structural biology?
-
Or scaling up a cost-sensitive diagnostic antigen?
Then try to define the required glycosylation profile suitable for different expression systems:
-
CHO or HEK293 cells for producing biologics requiring human-like N- and O-glycosylation, especially antibodies and membrane proteins
-
Insect or yeast systems to balance yield, cost, and functional glycosylation for vaccine subunits or industrial enzymes
-
E. coli for rapid, high-yield expression of non-glycosylated proteins, or as a synthetic chassis for engineered glycosylation pathways
-
Scalability, reproducibility, and regulatory acceptance as additional key factors—especially if moving toward clinical translation
Finally ensure feasibility, assess production scale, cost, timeline, and regulatory fit early in the project:
Larger-scale therapeutic applications may favor CHO systems for their regulatory precedent and lot-to-lot consistency, while microbial systems like Pichia or E. coli offer faster turnaround and lower costs for early discovery or diagnostic use. If clinical translation is the goal, selecting a host with a strong safety and regulatory track record becomes essential.
Expression System Selection Guide Table
The following section offers a guided comparison to help you make the right choice.
|
Choose Your Goal
|
Recommended System(s)
|
Glycosylation Need
|
|
Human therapeutic antibodies
|
CHO, HEK293
|
Human-like N-/O-glycans with Fc control
|
|
Structural biology of enzymes (non-glycosylated)
|
E. coli, Pichia pastoris (mutant lines)
|
None or simplified glycosylation
|
|
Subunit vaccines (e.g. viral proteins)
|
Insect cells, Pichia pastoris
|
Functional but not necessarily human-like
|
|
Diagnostic antigens
|
E. coli, S. cerevisiae
|
Minimal or controlled glycosylation
|
|
Glycoengineering research
|
CHO, Pichia, E. coli
|
Custom pathway design
|
|
Membrane proteins with O-glycosylation
|
HEK293, CHO
|
Complex O-glycan profiles
|
Still unsure? Creative Biolabs offers free project consultations to match your expression system with your glycoprotein goals.
Advantages & Limitations of Major Glycosylation Expression Systems
|
Solutions You Can Trust
|
Expression System
|
Advantages
|
Limitations
|
|
CHO & HEK293 Mammalian Glycoengineering
|
CHO Cells
|
-
Human-like N- and O-glycosylation patterns
-
Widely accepted by regulatory agencies
-
Stable expression lines for long-term use
-
Supports complex glycoengineering strategies
|
-
Higher production cost due to complex culture media
-
Slower cell doubling time compared to microbial systems
-
May require extensive downstream purification
|
|
HEK293 Cells
|
-
High transfection efficiency for transient expression
-
Capable of producing human-type glycoforms
-
Useful for membrane and secreted proteins
-
Ideal for early-stage screening
|
-
Less scalable for industrial production
-
Lower yield than CHO in stable expression
-
Greater variability in glycan profiles between batches
|
|
Insect Cell Glycosylation Optimization
|
Insect Cells
|
-
High protein yield
-
Scalable and cost-effective culture conditions
-
Suitable for viral proteins and VLPs
-
Baculovirus system enables large gene inserts
|
-
Lack of terminal sialylation
-
N-glycans are typically paucimannose-type
-
May induce immunogenic responses in humans
|
|
Yeast-Based Expression Customization
|
Pichia pastoris
|
-
Rapid growth and high cell density
-
Low-cost, chemically defined media
-
Strong inducible promoters
-
Amenable to glycosylation pathway engineering
|
-
Native glycosylation results in hypermannosylated N-glycans
-
Unmodified strains produce immunogenic glycans in humans
-
Limited capability for complex O-glycans
|
|
S. cerevisiae
|
-
Easy genetic manipulation
-
Fast and inexpensive culturing
-
High-level expression of secreted proteins
-
Well-established protocols and vectors
|
-
Produces long, branched high-mannose structures
-
Not suitable for therapeutic protein production without glycoengineering
-
Poor mimicry of mammalian glycosylation
|
|
Bacterial Glycosylation Pathway Engineering
|
E. coli
|
-
Extremely fast growth rate and protein expression
-
Well-characterized host with many tools available
-
Ideal for non-glycosylated proteins or synthetic glycosylation
-
Cost-effective and scalable
|
-
No natural glycosylation machinery
-
Requires engineered pathways to introduce glycosylation
-
Improper folding for some eukaryotic proteins
|
|
Plant Cell Glycosylation Solutions
|
Plant Cells
|
-
Capable of producing complex N-glycans with xylose and fucose modifications
-
Suitable for large-scale, low-cost biomass production
-
Low risk of human pathogen contamination
-
Amenable to human-like glycoengineering via gene editing
|
-
Native plant glycans may be immunogenic in humans
-
Longer development timelines compared to microbial systems
-
Limited industrial adoption for regulated therapeutics
|
Deliverables Provided in Each Project
Creative Biolabs offers a complete solution, from expression design to final product delivery. Clients receive detailed documentation and high-quality biomaterials ready for downstream use.
-
Expression system selection report based on target protein and glycosylation needs
-
Purified recombinant protein with certificate of analysis (CoA)
-
Customized glycosylation profile report (e.g., LC-MS, MALDI-TOF, SDS-PAGE)
-
Optional services including bioactivity testing, deglycosylation, and formulation
-
Glycoengineered cell lines or microbial strains, when applicable
What Makes Creative Biolabs the Right Partner?
With several years of experience, Creative Biolabs combines deep glycoscience expertise with industrial scalability, offering clients precision and flexibility across host systems. We're not just a service provider, we are your strategic partner in glycoengineering. Our competitive edge includes but not limited to:
-
Decades of Glycosylation Expertise: From therapeutic antibodies to microbial glycoproteins.
-
Multi-Platform Glycoengineering: Covering mammalian, insect, yeast, bacterial, and plant systems.
-
Custom Cell Line Development: Enable targeted glycoform optimization using knockdown, knock-in/out, overexpression or pathway reconstruction
-
High-Resolution Analytics: LC-MS/MS, HPAEC-PAD, MALDI-TOF, SDS-PAGE with glycan staining.
-
End-to-End Integration: From gene design → glycan optimization → expression → purification → QC.
-
Global Client Support: CRO experience serving pharma, biotech, and academic labs across continents.
Whether you're optimizing glycosylation for enhanced therapeutic efficacy, minimizing immune response in vaccines, or producing diagnostic enzymes at scale, Creative Biolabs offers bespoke expression system solutions. Contact us today to discuss your glycoprotein project with one of our specialists.
References
-
Sookhoo, Jamie RV, et al. "Protein Expression Platforms and the Challenges of Viral Antigen Production." Vaccines 12.12 (2024): 1344. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/vaccines12121344
-
Schütz, Anja, et al. "A concise guide to choosing suitable gene expression systems for recombinant protein production." STAR protocols 4.4 (2023): 102572. https://doi.org/10.1016/j.xpro.2023.102572
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 The workflow of mammalian expression system.1
Fig.1 The workflow of mammalian expression system.1