Defining Aldose and Ketose
Living organisms depend on carbohydrates which function both as essential biomolecules that provide primary energy sources and structural components. Monosaccharides represent the simplest sugars and they fall into two main groups depending on their carbonyl functional group placement which includes aldoses and ketoses. The Lobry de Bruyn–van Ekenstein transformation enables aldoses and ketoses to interchange forms through a natural chemical process which transforms sugars such as glucose into fructose and back again when proper conditions are met. The body's carbohydrate metabolism relies on this adaptability particularly within the pentose phosphate pathway as sugars transform continuously to fulfill cellular energy requirements and biosynthetic functions. To support your research on aldose and ketose, Creative Biolabs has developed advanced glycan analysis technologies, monosaccharides analysis for precise sugar profiling, and custom monosaccharide synthesis for tailored sugar production.
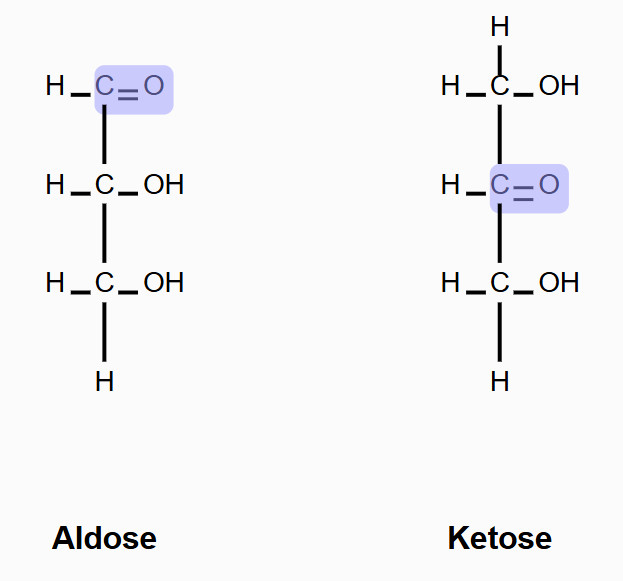 Fig.1 The structure of aldose and ketose.
Fig.1 The structure of aldose and ketose.
What is an Aldose?
Monosaccharides known as aldoses possess an aldehyde functional group at their terminal carbon atom which is carbon number one. Its general formula is Cn(H2O)n, where n≥3. Glyceraldehyde (C₃H₆O₃) represents the most basic form of all aldoses. Other Examples include glucose, galactose, and ribose. Aldoses are types of polyhydroxy aldehydes which contain multiple hydroxyl groups (-OH).
-
Aldehyde group at the terminal carbon.
-
Forms hemiacetal structures in cyclic forms.
-
Common in plants (e.g., glucose from photosynthesis).
-
Glucose is the primary energy source for cells.
-
Ribose is an essential component of RNA, DNA, and ATP.
-
Galactose is a crucial sugar in glycoproteins and glycolipids.
What is a Ketose?
A ketose is a monosaccharide that contains a ketone functional group (C=O) typically at the second carbon (C2) instead of at the terminal position. The simplest ketose is dihydroxyacetone (C₃H₆O₃), which lacks chirality. Examples include fructose, ribulose, and sorbose.
-
Ketone group within the carbon chain.
-
Forms hemiketal structures in cyclic forms.
-
Predominantly found in processed foods (e.g., high-fructose corn syrup).
-
Fructose is a naturally occurring sugar found in fruits and is metabolized in the liver.
-
Ribulose plays a role in the Calvin Cycle during photosynthesis.
Structural Differences Between Aldoses and Ketoses
Aldoses and ketoses differ fundamentally because of their carbonyl group placement. Their distinct reactivity and stereochemical properties along with biological roles are determined by this difference. Creative Biolabs provides structure analysis services for glycoprotein to uncover glycan structures and modifications that play essential roles in biomedical applications such as drug delivery systems and nanomaterial development.
|
Feature
|
Aldose
|
Ketose
|
|
Functional Group
|
Aldehyde (-CHO) at C1
|
Ketone (C=O) at C2
|
|
Example
|
Glucose, Galactose, Ribose
|
Fructose, Ribulose, Xylulose
|
|
Chiral Centers
|
More than ketoses of the same size
|
Fewer chiral centers
|
|
Mutarotation
|
Yes, forms α- and β-anomers
|
Yes, but slightly different process
|
|
Isomerization
|
Can convert into ketoses under alkaline conditions
|
Can convert into aldoses via enediol formation
|
Classification of Monosaccharides as Aldoses or Ketoses
Monosaccharides are classified based on two criteria:
-
Type of Carbonyl Group → Aldose vs. Ketose
-
Number of Carbon Atoms → Triose (3C), Tetrose (4C), Pentose (5C), Hexose (6C), etc.
|
Aldose Monosaccharides
|
Ketose Monosaccharides
|
|
Number of Carbons
|
Example
|
Number of Carbons
|
Example
|
|
3 (Triose)
|
Glyceraldehyde
|
3 (Triose)
|
Dihydroxyacetone
|
|
4 (Tetrose)
|
Threose
|
4 (Tetrose)
|
Erythrulose
|
|
5 (Pentose)
|
Ribose
|
5 (Pentose)
|
Ribulose
|
|
6 (Hexose)
|
Glucose
|
6 (Hexose)
|
Fructose
|
How to Identify Whether a Monosaccharide is an Aldose or a Ketose
To classify a monosaccharide as an aldose or a ketose, follow these steps:
-
Identify the Carbonyl Group:
-
If the carbonyl group is at C1, it is an aldose.
-
If the carbonyl group is at C2, it is a ketose.
-
Count the Carbon Atoms:
-
If it has three carbons (triose), it is either glyceraldehyde (aldose) or dihydroxyacetone (ketose).
-
If it has six carbons (hexose), it could be glucose (aldose) or fructose (ketose).
-
Check for Common Sugar Names:
-
Glucose, Galactose, Ribose, Mannose → Aldoses
-
Fructose, Ribulose, Xylulose → Ketoses
Applications of Aldoses and Ketoses
Aldoses and ketoses, due to their distinct chemical properties, have widespread applications in the food industry, pharmaceuticals, biotechnology, and beyond. The following analysis, based on recent research and technological advancements, highlights key developments in these domains.
Medical Applications
(1) Aldose Reductase Inhibitors
-
Epalrestat: A selective aldose reductase inhibitor that prevents glucose from being converted into sorbitol, mitigating complications such as diabetic neuropathy and retinopathy.
-
Ranirestat: A third-generation inhibitor currently in clinical trials, showing promise in improving renal function in diabetic nephropathy patients.
(2) Antiviral and Anticancer Agents
-
Ribavirin: A nucleoside analog derived from ribose (an aldose), ribavirin inhibits HCV RNA polymerase and is used in combination with interferon therapy for hepatitis C.
-
Floxuridine: A deoxyribose-derived anticancer drug that interferes with DNA synthesis, primarily used in colorectal cancer treatment. It is manufactured by Pfizer.
(3) Diagnostic Reagents
-
Fructose-¹³C Tracers: Isotope-labeled ketoses are employed in metabolic tracking for early liver cancer diagnosis.
-
Seliwanoff's Reagent: A colorimetric assay differentiating fructose (cherry-red color) from glucose (light pink), widely used for detecting adulterated honey.
Creative Biolabs provides high-throughput glycan screening services to support glycan biomarker discovery, facilitating advancements in diagnostics and therapeutic development.
Biotechnology and Industrial Enzymes
(1) Enzymatic Synthesis
-
L-Rhamnulose-1-Phosphate: Produced via rhamnose kinase and isomerase pathways, this compound serves as a precursor for antibiotics like neomycin.
-
D-Xylulose: Manufactured using xylose isomerase, D-xylulose is a key intermediate in riboflavin (vitamin B₂) synthesis.
(2) Biofuels
-
Cellobiose: A disaccharide derived from cellulose hydrolysis, cellobiose is fermented to produce bioethanol. POET, a U.S.-based company, commercializes this process using corn stover. With a commitment to sustainable innovation, Creative Biolabs also provides polysaccharides analysis for optimizing biofuel production and carbohydrate-based materials.
(3) Environmental Technology
-
Engineered Pseudomonas Strains: These bacteria metabolize ketoses to degrade petroleum pollutants, playing a crucial role in marine oil spill remediation.
Emerging Frontier Applications
(1) Nanomaterials
-
Glucose-based hydrogels: Aldose hydroxyl groups facilitate crosslinking, enabling controlled drug release in insulin-loaded nanoparticles.
(2) Artificial Sweetness Receptors
-
Fructose-binding proteins (FRUCTO-1): Designed based on fructose recognition mechanisms, these receptors enable the development of non-caloric sweetener molecules.
(3) Space Foods
-
Galactitol: A sugar alcohol derived from galactose, galactitol functions as a stabilizer for high-energy astronaut foods. NASA has successfully incorporated it into International Space Station (ISS) nutrition programs.
Monosaccharides that are aldoses or ketoses serve as essential components for biological activities and functions besides providing energy and forming structural elements in biomolecules. Biological functions of these compounds are determined by variations in carbonyl placement combined with stereochemistry and reactivity differences. The determination and categorization of sugars into aldoses or ketoses holds crucial importance for the fields of biochemistry, nutrition, and medicine. By integrating cutting-edge glycan analysis technologies and glycoengineering expertise, Creative Biolabs empowers researchers to drive innovations in carbohydrate-related applications. Explore our full suite of glycan synthesis services and carbohydrate analysis services to accelerate your research today.
Published Data
One study investigates how Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS) can be used to analyze carbohydrates and glycoconjugates. The study investigates various carbohydrate types including oligosaccharides, polysaccharides, glycoproteins, and glycolipids to demonstrate their importance across medical applications, industrial processes, and natural product development. MALDI-MS proves to be an optimal method for carbohydrate analysis because it produces single molecular ions which makes spectral interpretation easier and increases analytical precision. The ability of this approach to identify structural isomers like aldoses and ketoses stands out as its most important feature. The ion fragments produced from monosaccharides derivatized with 1-naphthaleneacethydrazide (NAH) during MALDI analysis are displayed in Figure 2. The derivatization of glucose produces specific ions with m/z values of 265 and 143 while fructose produces ions at m/z 295 and 119. The unique fragmentation patterns observed act as precise markers for distinguishing between aldoses and ketoses which enhances both sensitivity and specificity during carbohydrate analysis using MALDI-MS techniques.
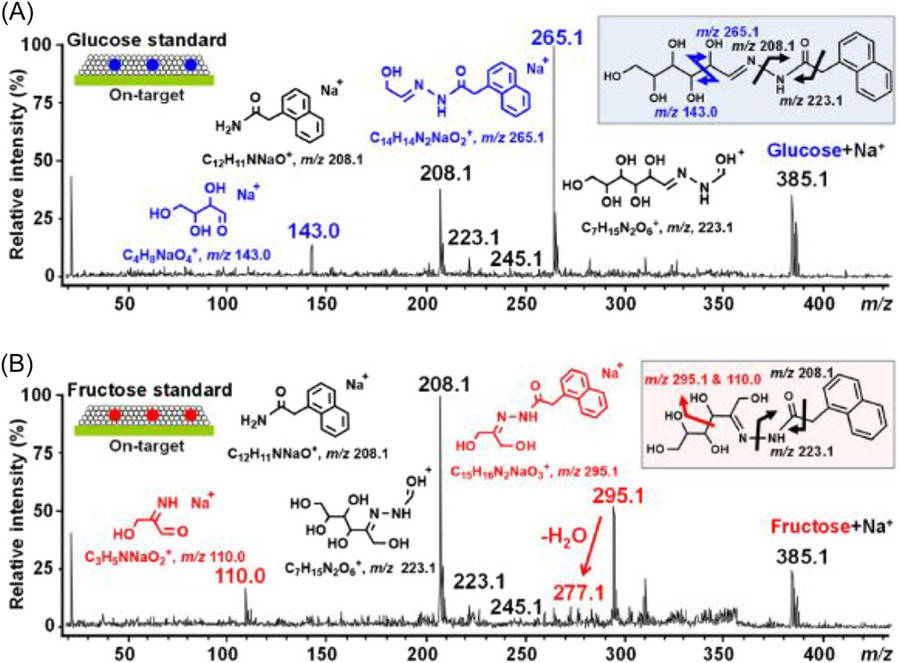 Fig.2 Specific ion fragments of NAH-derivatized monosaccharides in MALDI analysis.1
Fig.2 Specific ion fragments of NAH-derivatized monosaccharides in MALDI analysis.1
FAQs
Q: What are an aldose and a ketose?
A: An aldose is a monosaccharide with an aldehyde (-CHO) group at C1, while a ketose has a ketone (C=O) at C2. Examples include glucose (aldose) and fructose (ketose). They interconvert via isomerization reactions.
Q: How does ketose become aldose?
A: Ketoses convert to aldoses via the Lobry de Bruyn–Alberda van Ekenstein transformation, an isomerization catalyzed by bases or enzymes (e.g., aldose-ketose isomerases), shifting the carbonyl group from C2 to C1 through an enediol intermediate.
Q: What technology can be used to distinguish aldose and ketose?
A: Aldoses and ketoses can be distinguished using various analytical techniques. Seliwanoff's test is a colorimetric assay where ketoses react faster with resorcinol-HCl, producing a deep red color, while aldoses yield a lighter pink. Thin-layer chromatography (TLC) separates them based on polarity and mobility, while nuclear magnetic resonance (NMR) spectroscopy identifies structural differences in carbonyl positioning. Mass spectrometry (MS) differentiates based on molecular fragmentation patterns, and high-performance liquid chromatography (HPLC) resolves them by retention time using specialized columns.
References
-
Harvey, David J. "Analysis of carbohydrates and glycoconjugates by matrix‐assisted laser desorption/ionization mass spectrometry: An update for 2021–2022." Mass Spectrometry Reviews (2024). Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1002/mas.21873
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 The structure of aldose and ketose.
Fig.1 The structure of aldose and ketose.
 Fig.2 Specific ion fragments of NAH-derivatized monosaccharides in MALDI analysis.1
Fig.2 Specific ion fragments of NAH-derivatized monosaccharides in MALDI analysis.1

