Chromatographic methods remain the cornerstone of monosaccharide analysis due to their precision in resolving complex carbohydrate mixtures. High-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) dominate this field, offering complementary advantages for structural and quantitative studies.
HPLC based Monosaccharide Separation and Detection
Modern HPLC systems leverage specialized columns (e.g., C18 for hydrophobic interactions, DEAE-cellulose for anion exchange) and detectors like UV, refractive index (RI), and evaporative light-scattering detectors (ELSD) to resolve monosaccharides. The choice of column and mobile phase significantly impacts separation efficiency. Amino-bonded columns (e.g., NH2) and hydrophilic interaction liquid chromatography (HILIC) columns are widely used for resolving structurally similar monosaccharides like glucose and galactose. For instance, NH2 columns leverage differences in polarity and hydrogen bonding to separate these isomers in glycoprotein hydrolysates. Mobile phase optimization often involves acetonitrile/water gradients with additives such as phosphate buffers. A study on plant polysaccharides demonstrated that isocratic elution with 82% acetonitrile and 18% phosphate buffer (pH 6.8) achieved baseline separation of seven monosaccharides, including arabinose and xylose.
|
HPLC
|
High-resolution monosaccharide separation and quantification.
|
|
RP-HPLC
|
Enhanced glycan profiling with reversed-phase separation.
|
|
UHPLC/FLD/Q-TOF
|
Ultra-sensitive glycan analysis with fluorescence and mass detection.
|
Derivatization is critical for enhancing detection sensitivity. The use of 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatization is essential to improve detection sensitivity because monosaccharides without derivatization do not have UV-absorbent chromophores. Refractive Index (RI) detectors serve underivatized sugars while fluorescence detectors are appropriate for detecting labels such as 2-aminobenzamide (2-AB). The integration of PMP derivatization with UPLC-PDA systems produces detection limits down to 2.01 μg/mL which proves suitable for trace monosaccharide analysis of biologic samples. Thermal stability of polar monosaccharides can be achieved through derivatization protocols such as trimethylsilylation (TMS) and methoxyamination. TMS derivatives allow for the analysis of ribose in RNA hydrolysates because they increase volatility and protect against thermal degradation. Creative Biolabs develops reaction conditions that both reduce side reactions and achieve derivatization efficiency exceeding 95%.
GC-MS for Monosaccharide Composition Analysis
GC-MS excels in analyzing volatile monosaccharide derivatives, requiring hydrolysis and acetylation to stabilize sugars like glucose and rhamnose. Boronic acid-modified nanopore sensors coupled with GC-MS enable chiral differentiation of D/L isomers, such as distinguishing D-glucose from L-rhamnose in microbial polysaccharides. Recent advancements in permethylation protocols allow GC-MS to analyze insoluble polysaccharides like cellulose, overcoming limitations of traditional derivatization methods. Creative Biolabs offers high-sensitivity GC-MS for precise glycan profiling and isomer differentiation.
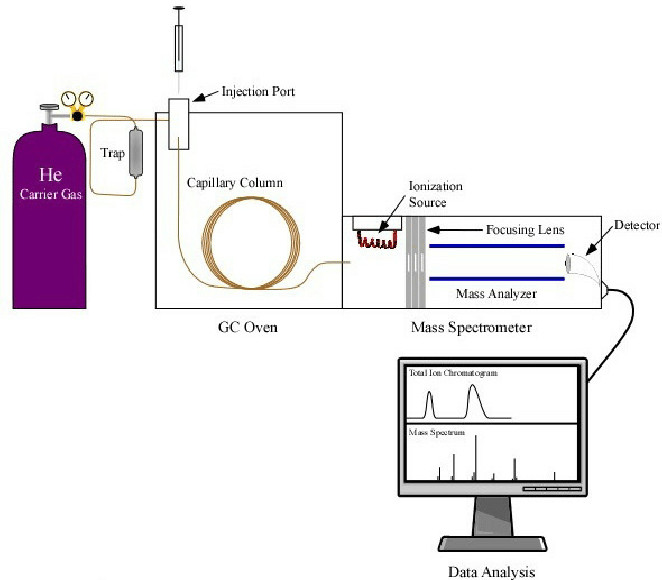 Fig.1 GC-MS Detection.1
Fig.1 GC-MS Detection.1
Advanced Spectroscopic and Mass Spectrometry Approaches
Spectroscopic and mass spectrometric techniques provide unparalleled insights into monosaccharide structure and glycosidic linkages, essential for understanding biological activity.
NMR Spectroscopy for Monosaccharide Structure Determination
Multidimensional NMR (e.g., HSQC, COSY) identifies anomeric configurations and rare sugars like N-acetylneuraminic acid. Quantitative 2D gsHSQC allows direct analysis of underivatized mixtures, as demonstrated in bacterial polysaccharide vaccines, where NMR confirmed O-acetylation patterns critical for immunogenicity. This non-destructive method is indispensable for validating glycan structures in therapeutic glycoconjugates.
|
FTIR
|
Fourier-transform infrared spectroscopy for glycan analysis.
|
|
NMR
|
Nuclear magnetic resonance spectroscopy for glycan structure analysis.
|
Mass Spectrometry in Monosaccharide Identification
Soft ionization techniques like MALDI-TOF and ESI-MS enable molecular weight profiling and linkage analysis. PMP-labeled UPLC-MS/MS, for example, simultaneously determines monosaccharide types and absolute configurations in Polyporus umbellatus polysaccharides, achieving picomolar sensitivity. Emerging strategies combining methylated tags with tandem MS now map glycosidic linkages in plant cell walls, resolving previously ambiguous structural motifs.
|
MS
|
High-resolution mass spectrometry for glycan analysis.
|
|
LC-ESI-MS
|
Liquid chromatography-electrospray ionization MS for detailed profiling.
|
|
MALDI-TOF MS
|
Rapid glycoprotein characterization via MALDI-TOF MS.
|
|
Imaging MS
|
Spatial glycan distribution analysis using MS imaging.
|
Sample Preparation and Derivatization Strategies
Robust sample preparation ensures accurate monosaccharide profiling, particularly for labile or low-abundance sugars.
Hydrolysis and Derivatization Protocols
Controlled acid hydrolysis with H2SO4 or trifluoroacetic acid (TFA) minimizes degradation of uronic acids and sialic acid. Ampule-sealed acidolysis preserves serum glycan integrity, enabling PMP-HPLC detection of sialylated biomarkers like Neu5Ac in cancer studies. Novel non-toxic reagents like 2-picoline borane further improve derivatization safety and efficiency.
Purification and Enrichment Techniques
Gel filtration (Sephadex) and ion-exchange chromatography (DEAE-cellulose) remove interfering compounds from polysaccharide hydrolysates. For example, Superdex columns isolated bioactive sulfated galactans from Gracilaria lemaneiformis, enhancing downstream NMR and MS analysis. Solid-phase extraction (SPE) cartridges tailored for carbohydrates now achieve >95% recovery of rare sugars like apiose.
Applications in Biomedical Research and Disease Studies
Monosaccharide analysis bridges structural biology and clinical diagnostics, revealing glycan signatures in disease.
Glycoprotein and Glycolipid Analysis in Disease Biomarkers
Aberrant sialylation (e.g., elevated Neu5Gc) in cancer glycoproteins correlates with metastasis, detectable via nanopore sensors that monitor galactose release during neuraminidase activity. In influenza research, ClyA nanopores quantify viral neuraminidase inhibition, guiding antiviral drug design.
Polysaccharide Structure-Activity Relationships in Drug Discovery
Sulfated fucoidans and β-glucans exhibit immunomodulatory effects dependent on monosaccharide composition. PMP-UPLC-MS analysis of Polyporus umbellatus polysaccharides revealed that low-molecular-weight fractions (5.97 kDa) activate the Nrf2/HO-1 pathway, mitigating oxidative stress in aging models. Similarly, antidiabetic polysaccharides from Liriope spicata show glucose-lowering effects linked to fructose-galactose ratios.
Emerging Technologies and Future Directions
Interdisciplinary innovations are reshaping monosaccharide analysis, enabling high-throughput and multi-omic integration.
Machine Learning and High-Throughput Screening
AI algorithms classify GC-MS data with 96% accuracy, distinguishing nine monosaccharides using MspA-PBA nanopores. Machine learning also optimizes hydrolysis conditions, reducing experimental time from weeks to hours.
Integration of Multi-Omics Platforms
Combining glycomics with proteomics and metabolomics unravels complex glycosylation networks. LC-MS/MS-based glycosite mapping in monoclonal antibodies, for instance, correlates galactosylation levels with antibody-dependent cellular cytotoxicity (ADCC). Such integrative approaches are pivotal for personalized medicine and biomarker discovery.
Creative Biolabs' Integrated Solutions
To choose the right monosaccharide identification methods, you may consider the following three pillars:
-
Structural resolution (anomericity, rare sugar detection, linkage analysis)
-
Operational throughput (batch processing vs single-molecule precision)
-
Application focus (QC compliance vs exploratory research)
HPLC – Speed and Specificity for Industrial Applications
HPLC-based heparin batch testing workflow efficiently processes up to 200 samples per day, with a detection threshold as low as 0.1% for contaminant glucuronic acid. Advanced UPLC-MS/MS platforms provide a balance between speed and specificity, making them well-suited for high-throughput industrial applications. A notable example is the CMED-derivatization method, which employs L-cysteine methyl ester tagging to achieve:
-
Simultaneous determination of D/L configurations – Critical for heparin quality control.
-
Comprehensive coverage of 26 monosaccharides, including uronic acids and sugar alcohols.
-
High recovery rates (98.53–102.13%) in plant polysaccharide analysis.
GC-MS – Unmatched Sensitivity for Detailed Structural Analysis
In microalgae EPS research, GC-MS enables precise detection of trace methylpentoses such as rhamnose at concentrations below 0.01%, which is crucial for monitoring salinity-induced sugar composition shifts. Although GC-MS requires time-intensive derivatization (e.g., trimethylsilylation), it remains the gold standard for high-sensitivity analysis:
|
Parameter
|
GC-MS
|
UPLC-MS/MS
|
|
Detection Limit (LOD)
|
0.1 pmol
|
1 pmol
|
|
Derivatization
|
Required
|
Optional
|
|
Isomer Separation
|
0.98 RSD
|
0.95 RSD
|
|
Throughput
|
40 samples/day
|
150 samples/day
|
In the rapidly evolving field of glycomics, monosaccharide analysis methods serve as the cornerstone for understanding carbohydrate-driven biological processes. At Creative Biolabs, we leverage cutting-edge glycan analysis techniques to decode sugar signatures in therapeutic antibodies, vaccine candidates, and natural polysaccharides. We offer end-to-end monosaccharide analysis services:
Published Data
The following figure shows that PMP-labeled HPLC successfully resolved neutral and acidic monosaccharides in Scrophularia ningpoensis polysaccharides, achieving baseline separation of 12 monosaccharides within 180 minutes. This method is particularly valuable for plant-derived polysaccharides, in which acidic sugars like glucuronic acid complicate analysis. Another study combines stable isotope tracing with metabolomics to directly observe the allocation of glucose to cell membrane glycans. This study showed the detection quantification of cell membrane monosaccharides. The study extracted monosaccharides from cell membranes using acid hydrolysis with TFA or acetic acid, followed by derivatization with PMP to enable separation and identification via LC-MS. The analysis successfully detected seven monosaccharides, including glucose, galactose, mannose, L-fucose, glucosamine, galactosamine, and N-acetylneuraminic acid. This method provides an accurate means to analyze the composition and abundance of monosaccharides in cell membrane glycans, offering a crucial tool for studying the structure and function of cell membrane glycans.
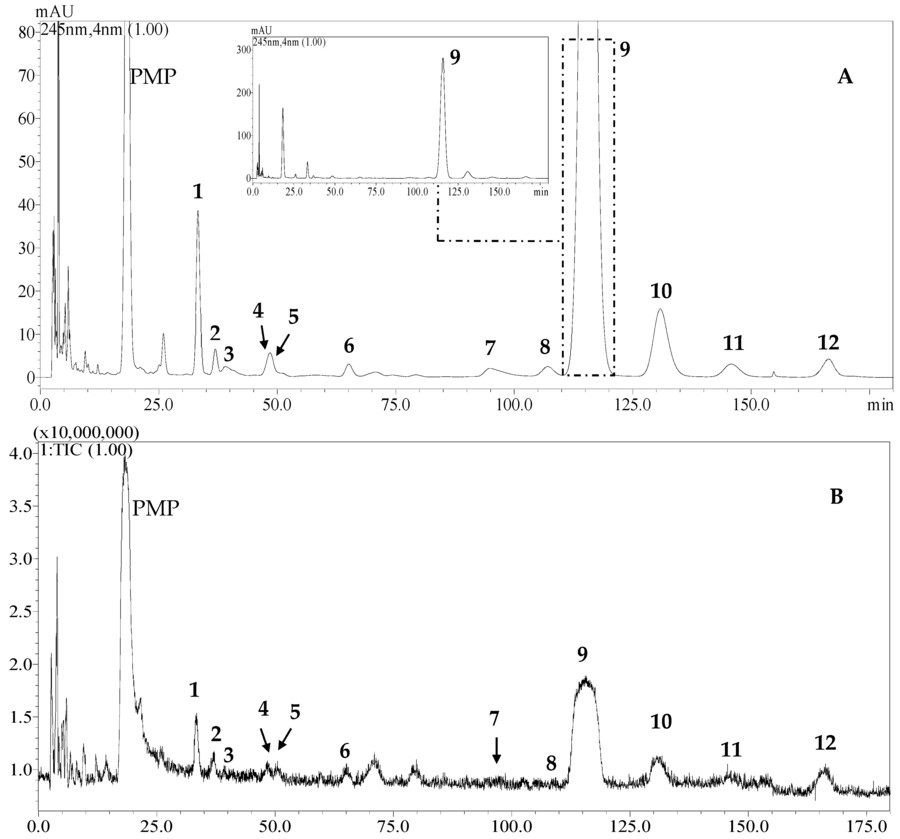 Fig.2 HPLC chromatogram (A) and positive mode TIC (B) of 12 PMP-derivatized monosaccharides.2
Fig.2 HPLC chromatogram (A) and positive mode TIC (B) of 12 PMP-derivatized monosaccharides.2
The evolution of monosaccharide analysis—from PMP-HPLC to AI-driven nanopores—has revolutionized glycobiology. At Creative Biolabs, we harness these technologies to accelerate therapeutic development, offering end-to-end solutions for glycan characterization and biomarker validation. As multi-omics and machine learning mature, the next decade promises unprecedented insights into the sweet secrets of life.
FAQs
Q1: How do you analyze monosaccharides?
A1: The analysis of monosaccharides employs chromatographic and spectrometric methods including HPLC and GC-MS alongside NMR spectroscopy and mass spectrometry. The analytical process requires sample hydrolysis followed by derivatization techniques such as PMP labeling for HPLC or TMS for GC-MS before detection through UV, fluorescence or MS methods. NMR spectroscopy along with high-resolution MS provides structural insights that reveal glycosidic linkages and anomeric configurations which facilitate complete profiling for biological and pharmaceutical studies.
Q2: What are the methods of determination of configuration of monosaccharides?
A2: The configuration of monosaccharides (D/L and α/β anomers) is determined using optical rotation measurements, chiral chromatography, and spectroscopic methods like NMR and circular dichroism (CD). High-performance HPLC and GC-MS with chiral derivatization (e.g., L-cysteine methyl ester tagging) can resolve enantiomers. NMR spectroscopy provides insights into anomeric configurations through chemical shift differences in ¹³C-NMR and coupling constants in ¹H-NMR, allowing precise structural assignment.
Q3: What are the three types of monosaccharides that are most commonly studied?
A3: Glucose, galactose, and mannose receive the most scientific attention because they function as key components in energy metabolism and participate in glycoproteins and cell signaling pathways. Cells use glucose as their main energy source while galactose serves essential functions in glycoproteins and glycolipids and mannose participates in the process of N-glycan biosynthesis. Medical research often examines these sugars because of their relevance to diabetes studies and cancer biomarker research as well as glycoprotein therapeutic investigations.
References
-
Wu, Shubin, Gaojin Lv, and Rui Lou. "Applications of chromatography hyphenated techniques in the field of lignin pyrolysis." Applications of Gas Chromatography. IntechOpen, 2012. Distributed under Open Access license CC BY 3.0, without modification. http://dx.doi.org/10.5772/32446
-
Guo, Ning, et al. "Quantitative analysis of polysaccharide composition in Polyporus umbellatus by HPLC–ESI–TOF–MS." Molecules 24.14 (2019): 2526. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/molecules24142526
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 GC-MS Detection.1
Fig.1 GC-MS Detection.1
 Fig.2 HPLC chromatogram (A) and positive mode TIC (B) of 12 PMP-derivatized monosaccharides.2
Fig.2 HPLC chromatogram (A) and positive mode TIC (B) of 12 PMP-derivatized monosaccharides.2