Biochemical monosaccharides serve as basic structural units with carbon chains of 3-7 atoms along with a carbonyl group, plus multiple hydroxyl groups. Creative Biolabs interprets complex natural structures through practical classification methods. Glyceraldehyde and other trioses act as vital metabolic intermediates but remain uncommon. Genetic material relies on pentoses such as ribose and deoxyribose for its backbone while hexoses like glucose and fructose support energy production and provide natural sweetness.
Creative Biolabs delivers simple monosaccharide solutions to advance research in drug discovery and vaccine development. Our company delivers custom synthesis for glycans that span trioses to heptoses and performs advanced glycan analysis via NMR, HPLC, and mass spectrometry techniques while executing precise glycan modifications and glycoconjugation services for glycoengineering. Our team excels at solving technical problems related to sugar chain orientation and functionalization to deliver customized solutions for optimizing glycoproteins and designing carbohydrate-based vaccines. Our practical approach helps scientific teams transform monosaccharide chemistry into meaningful biological discoveries by avoiding theoretical complexity.
Monosaccharide Examples
Monosaccharides are everywhere—in your DNA, your breakfast, and even cutting-edge therapies. They can be grouped into common types, including glucose, fructose, galactose, mannose, ribose and deoxyribose, and special types, such as raffinose-related sugars, rhamnose, and amino sugars, each with specific biological roles in energy storage, structural functions, or specialized metabolic pathways.
Common Types of Monosaccharides
Galactose, a glucose C4 epimer, shares the formula C6H12O6 but differs in the spatial orientation of its C4 hydroxyl group. This subtle structural distinction confers unique biological roles. As a component of lactose (linked to glucose via β-1,4-glycosidic bonds), galactose constitutes a primary energy source in mammalian milk. However, its metabolism requires conversion to glucose-6-phosphate via the Leloir pathway in the liver; defects in this pathway cause galactosemia, a disorder marked by toxic galactose accumulation. Beyond energy metabolism, galactose residues in glycoproteins and glycolipids mediate critical cellular interactions, such as immune cell recognition via galectin binding and neural development through glycosphingolipid synthesis. Its role in maintaining glycosylation patterns during nutrient scarcity further underscores its evolutionary significance.
Glucose reigns supreme as the universal energy currency. Humans metabolize it via glycolysis, generating ATP for everything from muscle contractions to brain function. Meanwhile, plants repurpose fructose into sucrose for energy storage in fruits—a clever strategy to entice seed dispersers. Mannose, another hexose, plays a dual role: it fuels cells and assists in glycoprotein synthesis. Immune cells use mannose-decorated proteins to recognize pathogens, acting like molecular barcodes. Not all monosaccharides are about energy. Ribose forms RNA's backbone, while its deoxygenated cousin, deoxyribose, stabilizes DNA. Arabinose, found in plant cell walls, acts as nature's glue, binding cellulose fibers into a robust matrix. Xylose, a pentose from agricultural waste, is fermented into xylitol (a tooth-friendly sweetener) or biofuels—turning crop residues into green energy.
Amino Monosaccharide
Amino monosaccharides may not get as much attention as glucose or fructose, but they are just as vital to life. These specialized sugars, which carry an amino group instead of a hydroxyl, are deeply embedded in the fabric of biology. They help build cartilage, shape immune responses, and even determine blood types. Found in everything from the shells of crustaceans to the protective mucus lining our intestines, they serve as the backbone of glycoproteins, glycolipids, and glycosaminoglycans (GAGs). Whether supporting joint health or playing a role in viral recognition, these sugars are more than just structural components—they are active players in the complex language of cellular communication. The table below breaks down some of the key amino monosaccharides, where they are found, and what makes them biologically important.
|
Amino Monosaccharide
|
Formula
|
Where to Find?
|
Biological Functions
|
|
Glucosamine
|
C₆H₁₃NO₅
|
Chitin in crustaceans, joint cartilage
|
Precursor for GAGs in cartilage and connective tissues; essential for hyaluronic acid and heparan sulfate synthesis; supports joint health.
|
|
Galactosamine
|
C₆H₁₃NO₅
|
Glycoproteins, glycosaminoglycans
|
Major component of chondroitin sulfate; involved in cartilage integrity and cellular signaling.
|
|
Mannosamine
|
C₆H₁₃NO₅
|
Sialic acid precursor in glycoproteins
|
Key intermediate in sialic acid biosynthesis, crucial for immune responses and cell adhesion.
|
|
N-Acetylglucosamine (GlcNAc)
|
C₈H₁₅NO₆
|
Chitin, bacterial cell walls, glycoproteins
|
Structural component of chitin in fungal cell walls; integral to O-linked and N-linked glycosylation in eukaryotic cells.
|
|
N-Acetylgalactosamine (GalNAc)
|
C₈H₁₅NO₆
|
Mucins, blood group antigens
|
Major component of mucins in epithelial linings; defines A-type blood group antigen.
|
|
N-Acetylneuraminic Acid (Sialic Acid, Neu5Ac)
|
C₁₁H₁₉NO₉
|
Cell membranes, glycoproteins, gangliosides
|
Critical for cell-cell communication, viral recognition (e.g., influenza binding), and neuronal plasticity.
|
Monosaccharide Types Overview
|
Monosaccharide
|
Formula
|
Aldoses or Ketoses?
|
Where to Find?
|
Biological Functions
|
|
Glucose
|
C₆H₁₂O₆
|
Aldose
|
Fruits, honey, animal blood, plant starch
|
Primary energy source via glycolysis and oxidative phosphorylation; intermediate in gluconeogenesis and glycogen metabolism; regulates blood sugar via insulin and glucagon. The main energy source for brain cells and red blood cells.
|
|
Fructose
|
C₆H₁₂O₆
|
Ketose
|
Fruits, honey, some vegetables
|
Metabolized in the liver via fructolysis; converted into glucose or fatty acids; used as a sweetener due to higher sweetness than sucrose. Found in high-fructose corn syrup used in soft drinks.
|
|
Galactose
|
C₆H₁₂O₆
|
Aldose
|
Milk, dairy products, plant polysaccharides
|
Component of lactose in milk; essential for glycolipid and glycoprotein synthesis in nerve cells; converted to glucose in the liver. A key sugar in infant nutrition via breast milk.
|
|
Mannose
|
C₆H₁₂O₆
|
Aldose
|
Cranberries, legumes, seaweed
|
Essential for N-glycosylation of proteins; recognized by mannose receptors in immune response; inhibits bacterial adhesion in the urinary tract. Used in dietary supplements to prevent UTIs.
|
|
Ribose
|
C₅H₁₀O₅
|
Aldose
|
Cells, RNA, ATP
|
Structural component of RNA and nucleotides like ATP, NADH, and FAD; essential for protein synthesis and cellular energy metabolism. Forms the sugar backbone of RNA molecules.
|
|
Deoxyribose
|
C₅H₁₀O₄
|
Aldose
|
DNA in chromosomes
|
Key structural component of DNA; lacks 2'-OH group, making DNA more stable than RNA; essential for genetic information storage. Present in all living organisms' chromosomes.
|
|
Xylose
|
C₅H₁₀O₅
|
Aldose
|
Plant hemicellulose, wood, corncobs
|
Precursor for xylitol, a sugar substitute used in diabetic-friendly foods; involved in the pentose phosphate pathway for nucleotide biosynthesis. Commonly used in chewing gum to prevent tooth decay.
|
|
Arabiose
|
C₅H₁₀O₅
|
Aldose
|
Plant gums, hemicellulose, pectin
|
Inhibits sucrase enzyme, reducing sucrose digestion and blood sugar spikes; promotes gut health by supporting beneficial bacteria. Added to functional foods to aid in weight management.
|
|
Rhamnose
|
C₆H₁₂O₅
|
Aldose
|
Plant polysaccharides, bacterial polysaccharides
|
Component of bacterial cell walls and plant glycosides; involved in immune recognition; contributes to bacterial virulence. Found in lipopolysaccharides of Gram-negative bacteria.
|
|
Allose
|
C₆H₁₂O₆
|
Aldose
|
Rare in nature, some plants
|
Rare sugar with potential anti-cancer and anti-inflammatory properties; used as a low-calorie sugar alternative. Studied for its effects in reducing oxidative stress in cells.
|
|
Talose
|
C₆H₁₂O₆
|
Aldose
|
Plants, microorganisms
|
Possesses antibacterial properties; mild diuretic effects; potential applications in metabolic regulation. Explored for therapeutic use in controlling diabetes.
|
|
Idose
|
C₆H₁₂O₆
|
Aldose
|
Plants, microorganisms
|
Participates in structural functions within biological systems; metabolic functions still under research. Found in specialized polysaccharides in certain plants and microbes.
|
|
Muramyl Sugar
|
C₁₉H₃₂N₄O₁₁
|
Derived Sugar
|
Bacterial cell walls
|
Recognized by the innate immune system; triggers macrophage activation and inflammation response; a target for antibiotic development. A key component of the bacterial peptidoglycan layer.
|
|
Apiose
|
C₅H₁₀O₅
|
Aldose
|
Plant pectin, secondary metabolites
|
Integral in plant cell wall polysaccharides; aids in boron fixation critical for plant growth; precursor in flavonoid biosynthesis. Present in parsley and celery cell walls.
|
|
Xylulose
|
C₅H₁₀O₅
|
Ketose
|
Hemicellulose degradation, metabolic byproducts
|
Intermediary in the pentose phosphate pathway; serves as a metabolic marker for certain disorders. Identified in individuals with pentosuria, a rare metabolic condition.
|
|
Xylonic Acid
|
C₅H₁₀O₆
|
Sugar Acid
|
Lignocellulose degradation, microbial fermentation
|
Used as a cement additive for alkali resistance; acts as a biocatalyst; employed in low-calorie sweeteners. Utilized in sustainable industrial processes for green chemistry.
|
Monosaccharides in Drug Development and Glycoengineering
At Creative Biolabs, we harness the transformative power of monosaccharides to advance modern medicine. Heparin, a sulfated polysaccharide derived from glucosamine and iduronic acid units, exemplifies how sugar-based molecules save lives by preventing blood clots. While traditional heparin carries bleeding risks, synthetic analogs like fondaparinux demonstrate how structural optimization minimizes side effects—a principle central to our carbohydrate engineering services. Monosaccharide modifications are revolutionizing biologics development. In antibody therapeutics, precise Fc-region glycosylation directly impacts drug efficacy. Obinutuzumab's 10-fold enhancement in ADCC activity, achieved through core fucose reduction, underscores the therapeutic value of glycoengineering—an area where our glycosylation profiling and customization services excel. Beyond mammalian systems, breakthroughs in plant-based production highlight sugar chain control: silencing plant-specific glycosyltransferases in Lemna minor yielded antibodies with human-like glycans, boosting therapeutic activity by 30% while reducing immunogenicity.
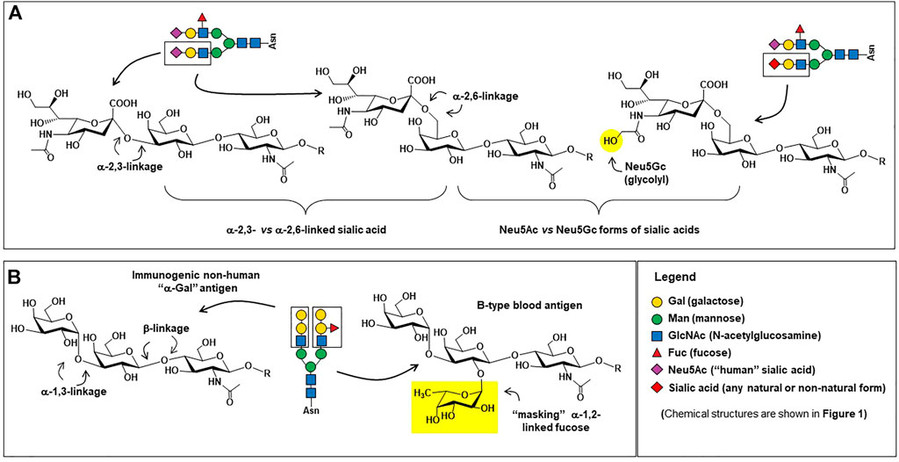 Fig.1 Therapeutic antibodies-related glycan epitopes.1
Fig.1 Therapeutic antibodies-related glycan epitopes.1
These cases align with our mission to deliver end-to-end monosaccharide solutions. From synthesizing heparin analogs to optimizing glycosylation patterns in antibody platforms, Creative Biolabs provides analytical tools, custom synthesis, and glyco-modification strategies that turn structural precision into clinical impact. Whether refining drug safety or enhancing biologics functionality, we empower researchers to master the sugar code of life. We blend nature's elegance with synthetic ingenuity to deliver:
Published Data
Metabolic glycoengineering enables precise modification of antibody glycans for targeted conjugation in antibody-drug conjugates (ADCs). The metabolic introduction of thiol-modified fucose proves superior among alternative methods due to its high efficiency and bio-orthogonal nature. The strategy integrates thiol-functionalized fucose into the Fc glycan structure of antibodies to enable conjugation through maleimide chemistry. The thiol functionality creates a highly selective and stable bond that enables accurate drug attachment while preserving the antibody's natural function. Monosaccharide modifications play a pivotal role in the development of glycoengineering-based therapeutic strategies. Periodate oxidation for aldehyde formation together with azido-sialic acid incorporation and alkyne-modified glycans for click chemistry provide supplementary methods for precise site-specific modifications. The combined use of these methods demonstrates how sugar modifications can transform bioconjugation processes and target drug delivery systems.
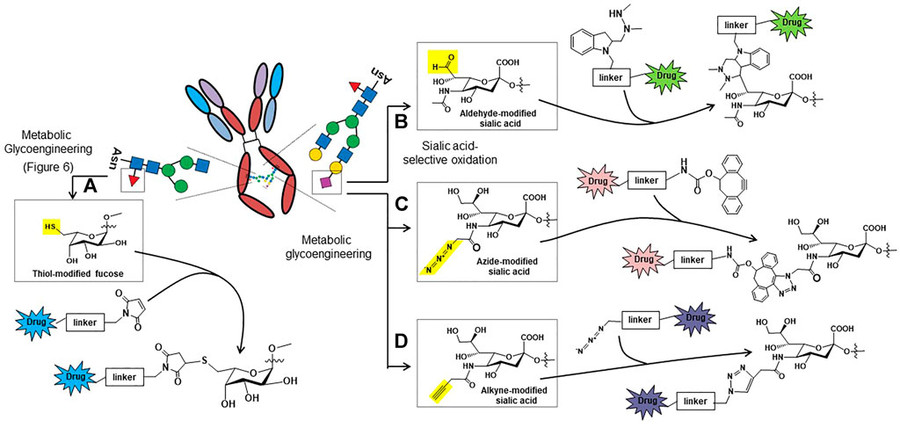 Fig.2 Glycosylation-based antibody-drug conjugation strategies.1
Fig.2 Glycosylation-based antibody-drug conjugation strategies.1
FAQs
Q: What are monosaccharides?
A: Monosaccharides are the simplest carbohydrates, serving as fundamental building blocks for oligosaccharides and polysaccharides. They are classified based on carbon number (trioses to hexoses) and functional groups (aldoses vs. ketoses). In biopharmaceuticals, monosaccharides play vital roles in glycoproteins, glycolipids, and cell signaling. At Creative Biolabs, we explore tailored sugar modifications for improved drug targeting, stability, and immunogenicity in glycoengineered therapeutics. (Glycan Modification and Labeling Services)
Q: How do you differentiate the three common monosaccharides?
A: Glucose, fructose, and galactose share the molecular formula C₆H₁₂O₆ but differ structurally. Glucose is an aldose with an aldehyde at C1, while fructose is a ketose with a ketone at C2. Galactose, an epimer of glucose, differs at C4. These structural variations impact metabolism and biological roles.
Q: How does the acetylation of monosaccharides occur?
A: Acetylation of monosaccharides typically involves enzymatic or chemical addition of an acetyl group (-COCH₃) to hydroxyl or amine functional groups. In biological systems, N-acetylation of glucosamine and galactosamine forms key glycans like N-acetylglucosamine (GlcNAc) and N-acetylgalactosamine (GalNAc), crucial for glycoproteins and mucins. Creative Biolabs leverages glycoengineering techniques to modify sugar acetylation, enhancing stability and functionality in biopharmaceutical applications.
Q: How to choose between HPLC and NMR for sugar analysis?
A: HPLC excels at separating mixtures (e.g., glucose vs. galactose), while NMR reveals structural details like anomer configuration. We'll tailor solutions to your needs!
Reference
-
Dammen-Brower, Kris, et al. "Strategies for glycoengineering therapeutic proteins." Frontiers in chemistry 10 (2022): 863118. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fchem.2022.863118
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Therapeutic antibodies-related glycan epitopes.1
Fig.1 Therapeutic antibodies-related glycan epitopes.1
 Fig.2 Glycosylation-based antibody-drug conjugation strategies.1
Fig.2 Glycosylation-based antibody-drug conjugation strategies.1

