Monosaccharides, the simplest form of carbohydrates, exhibit unique chemical properties driven by their functional groups: hydroxyl (-OH), aldehyde (-CHO), or ketone (C=O) groups. These features enable their wide applications across industries. In food industry, monosaccharides like fructose act as natural sweeteners and stabilizers in syrups and baked goods. While in pharmaceuticals design, chemical modification in monosaccharides help improve efficiency of drug delivery, boosting therapeutic efficacy. Creative Biolabs, equipped with specialized glycan analysis technologies and experienced expert staff, offers full services spanning from custom glycan synthesis, tailored carbohydrate analysis to high-quality glycoengineering platforms, facilitating your glycan-based research with expertise and passion!
Monosaccharide Chemical Reactivity
The multiple hydroxyl groups in monosaccharides, such as glucose and fructose, contribute to their high hydrophilicity. This property enables monosaccharides to dissolve readily in water, forming hydrogen bonds. Additionally, the presence of aldehyde (in aldoses like glucose) or ketone (in ketoses like fructose) groups grants monosaccharides reducing capabilities. For example, glucose can reduce metal ions in Fehling's or Benedict's solutions, a principle utilized in blood glucose test strips for diabetes monitoring. Monosaccharides also exist in a dynamic equilibrium between open-chain and cyclic forms (tautomerism). This structural flexibility influences their chemical behavior. For instance, glucose primarily adopts a pyranose (six-membered ring) structure in aqueous solutions, which stabilizes the molecule and affects its reactivity in glycosidic bond formation.
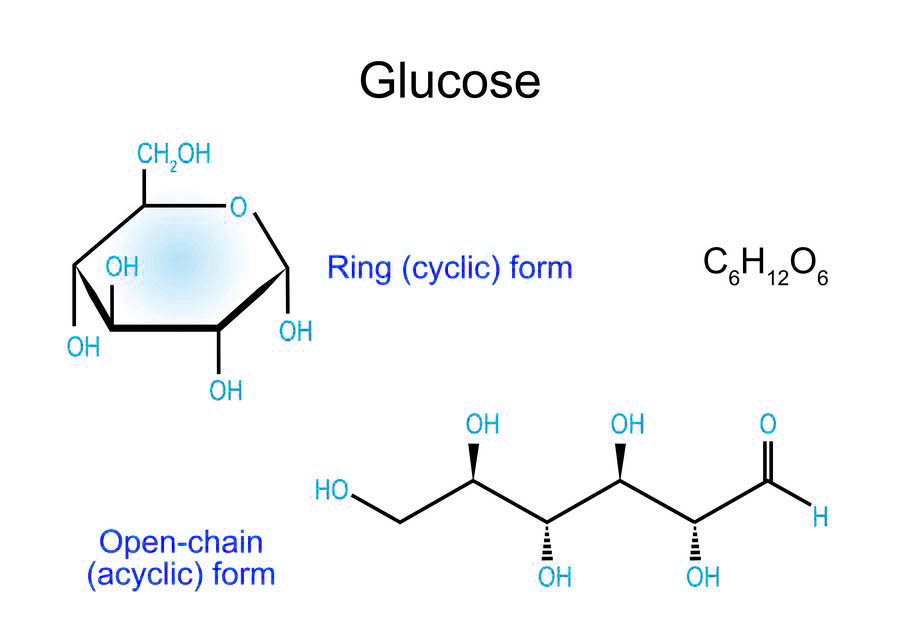 Fig.1 The ring form and open-chain form of glucose.
Fig.1 The ring form and open-chain form of glucose.
The distinct chemical behaviors of aldoses and ketoses are critical in industrial processes. Aldoses undergo oxidation more readily than ketoses. For example, glucose oxidizes to gluconic acid with mild oxidizing agents (e.g., bromine water), while stronger oxidants like nitric acid yield saccharic acid. Ketoses, such as fructose, require enzymatic or alkaline conditions to participate in oxidation reactions. Hydroxyl groups in monosaccharides can be chemically modified through esterification (e.g., phosphorylation) or etherification (e.g., methylation). Methylation of hydroxyl groups is a key step in analyzing carbohydrate structures via gas chromatography-mass spectrometry (GC-MS). In pharmaceuticals, phosphorylation of monosaccharides enhances their ability to cross cell membranes, making them ideal for drug delivery systems.
Chemical Reactions of Monosaccharides: Oxidation, Reduction, Glycosylation, and Beyond
Monosaccharides undergo diverse chemical transformations, enabling their use in industrial and biomedical applications. We expand on oxidation, reduction, glycosylation, ester formation, and fermentation.
Oxidation Reactions
Aldoses readily undergo oxidation to produce carboxylic acids. For instance, glucose oxidizes to gluconic acid using mild agents like bromine water. Strong oxidants (e.g., nitric acid) further convert aldoses to aldaric acids (e.g., glucaric acid), which serve as biodegradable chelators in detergents. In medical diagnostics, glucose oxidase enzymes on blood glucose test strips catalyze glucose oxidation, generating measurable signals for diabetes management.
Reduction Reactions
Reduction of monosaccharides yields sugar alcohols, such as sorbitol (from glucose) and xylitol (from xylose). These compounds are vital in sugar-free foods and oral care products due to their non-cariogenic properties. Xylitol, for example, inhibits bacterial growth in toothpaste formulations. Industrial-scale reduction processes often employ catalytic hydrogenation with nickel catalysts to achieve high purity (>95%).
Glycosidic Bond Formation
Glycosylation links monosaccharides via glycosidic bonds to form oligo- or polysaccharides. Enzymatic methods (e.g., glycosyltransferases) dominate industrial production due to their specificity. Sucrose synthesis, a disaccharide of glucose and fructose, relies on enzymatic processes to ensure efficiency and minimal byproducts.
Ester Formation
Monosaccharide hydroxyl groups can react with acids to form esters, such as phosphates or acetates. These esters derivates are widely existed in industrial production. For instance, glucose-6-phosphate is a critical intermediate in cellular metabolism. Synthetic phosphorylation of monosaccharides enhances their utility in drug delivery systems by improving membrane permeability. And acetylated sugars (e.g., sucrose acetate isobutyrate) act as emulsifiers in food additives or coatings for pharmaceuticals. Creative Biolabs offers custom monosaccharide synthesis (e.g., site-specific phosphorylation).
Fermentation
Monosaccharides are primary substrates in microbial fermentation, playing a crucial role in both biofuel production and industrial bioprocesses. Quantitative profiling in biogas fermentation enhances microbial analysis, optimizing biofuel yield. In industrial applications, glucose-rich biomass (e.g., corn starch) undergoes large-scale conversion to ethanol. Additionally, fermentation-derived monosaccharide derivatives (e.g., hyaluronic acid) serve as valuable components in drug formulations. Our biomass components quantitative profiling support more efficient biomass utilization, helping clients maximize resource efficiency. Common fermentation processes include:
-
Ethanol Production: Yeast ferments glucose to ethanol, a renewable biofuel.
-
Lactic Acid Synthesis: Bacterial fermentation of glucose yields lactic acid, used in biodegradable plastics (PLA) and food preservation.
-
Specialty Chemicals: Rare sugars like tagatose are produced via enzymatic fermentation of galactose, offering low-calorie sweetening solutions.
Physical Properties of Monosaccharides
The physical properties of monosaccharides, including solubility, crystallinity, and optical activity, are pivotal for their industrial utilization. At Creative Biolabs, we provide a suite of monosaccharide analysis services for precise optical purity verification.
Solubility and Crystallinity
Monosaccharides vary in solubility depending on their structure. Glucose is highly soluble in water, whereas mannose dissolves less readily. These differences influence their applications. In confectionery manufacturing, controlled crystallization of glucose ensures the smooth texture of hard candies. Crystallization processes are optimized based on solubility profiles. For example, fructose's high solubility and low crystallization tendency make it suitable for soft candies and liquid sweeteners.
Optical Activity and Chirality
Monosaccharides contain chiral centers, leading to optical isomerism (D- and L-forms). Biological systems predominantly recognize D-sugars, such as D-glucose, due to their compatibility with enzyme active sites. This specificity is exploited in drug design. For instance, L-glucose, a non-metabolizable form, is studied for potential use in low-calorie sweeteners.
Optical purity is critical in pharmaceutical quality control. Impurities in chiral monosaccharides can alter drug efficacy or safety. At Creative Biolabs, we employ high-performance liquid chromatography (HPLC) coupled with polarimetry to ensure ≥99% optical purity in monosaccharide-based APIs.
Applications of Monosaccharides
Monosaccharide derivatives play vital roles in vaccine adjuvants and antiviral therapies. QS-21, a saponin-based adjuvant extracted from tree bark, contains monosaccharide units that enhance immune responses in vaccines. Glycoarray technology, which uses monosaccharide-coated chips, enables rapid screening of disease biomarkers like cancer-associated glycans. Monosaccharides are precursors to hyaluronic acid, a popular moisturizer in skincare. Through molecular modification, monosaccharides like glucosamine improve hyaluronic acid's stability and hydration capacity. Additionally, monosaccharide-based surfactants (e.g., alkyl polyglucosides) are biodegradable alternatives to petroleum-derived detergents. We offer structural validation of glycosylated molecules using LC-MS and NMR.
Monosaccharides' unique chemical and physical properties underpin their versatility in food, pharmaceuticals, and biotechnology. At Creative Biolabs, we leverage advanced technologies—from enzymatic synthesis to chiral analysis—to deliver tailored monosaccharide solutions. Our services, including process optimization and structural validation, empower industries to innovate while ensuring quality and efficiency. Contact us for more monosaccharide-related services details.
FAQs
Q1: What are the properties of monosaccharides and polysaccharides?
A1: Monosaccharides are simple sugars that dissolve easily in water due to their hydroxyl groups, making them highly hydrophilic. They can also act as reducing agents if they have a free aldehyde or ketone group. In contrast, polysaccharides are long chains of sugar units with diverse properties—some, like starch and glycogen, store energy, while others, such as cellulose and chitin, provide structural support in plants and animals. Their solubility, digestibility, and function depend on how the sugar units are linked and arranged.
Q2: What is the function of the monosaccharide?
A2: Monosaccharides are the body's go-to energy source, with glucose playing a central role in fueling cells. Through glycolysis and cellular respiration, glucose is converted into ATP, the molecule that powers biological processes. But monosaccharides do more than just provide energy. They serve as building blocks for complex carbohydrates like glycogen and cellulose and are involved in producing nucleotides, glycoproteins, and glycolipids, which support cell signaling, immune responses, and structural integrity. In medicine and biotechnology, modified monosaccharides are even used in drug delivery and therapeutic applications.
Q3: What is the structure of monosaccharides?
A3: Monosaccharides have a simple yet flexible structure, consisting of a carbon backbone with multiple hydroxyl (-OH) groups and either an aldehyde or ketone functional group. Based on this, they are classified as aldoses or ketoses. In solution, they don't always stay in their linear form—most spontaneously fold into ring structures, known as furanose (five-membered) or pyranose (six-membered) rings. This ability to switch between structures affects how they interact with enzymes, form glycosidic bonds, and participate in metabolism, making them essential to both biology and industry.
Reference
-
Zhao, Ting, et al. "Structural modification and biological activity of polysaccharides." Molecules 28.14 (2023): 5416. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/molecules28145416
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 The ring form and open-chain form of glucose.
Fig.1 The ring form and open-chain form of glucose.

