Define Monosaccharide
Monosaccharides are the simplest form of carbohydrates, often referred to as "simple sugars." These molecules consist of a single sugar unit and cannot be broken down into smaller carbohydrates through hydrolysis. The term "monosaccharide" comes from the Greek monos (single) and sakcharon (sugar). Structurally, they are polyhydroxy aldehydes or ketones with the general formula CnH2nOn. While this formula might suggest a straightforward structure, monosaccharides can adopt complex cyclic forms that influence their biological activity. Monosaccharides serve as building blocks for disaccharides and polysaccharides, making them foundational to life processes. Creative Biolabs focuses on the definition, types, analytical methodologies, and biomedical applications of monosaccharides, with innovation platforms to advance research in monosaccharide research.
Monosaccharides & Disaccharides & Polysaccharides
Monosaccharides are directly absorbed into the bloodstream during digestion, whereas disaccharides and polysaccharides require enzymatic breakdown. This distinction highlights their role as immediate energy sources versus storage or structural molecules. Monosaccharides differ from disaccharides and polysaccharides in complexity and function:
|
|
Monosaccharides
|
Disaccharides
|
Polysaccharides
|
|
Chemical Formula
|
CₙH₂ₙOₙ (n=3-7)
|
C₁₂H₂₂O₁₁
|
(C₆H₁₀O₅)ₙ (n=40-3000)
|
|
Hydrolysis Products
|
Non-hydrolyzable
|
Hydrolyzes into two monosaccharides (e.g., sucrose → glucose + fructose)
|
Hydrolyzes into multiple monosaccharides (e.g., starch → glucose)
|
|
Biological Functions
|
- Direct energy source (e.g., glucose)
- Nucleotide component (e.g., ribose, deoxyribose)
|
- Energy transport (e.g., sucrose in plants)
- Specific functions (e.g., lactose as an energy source for infants)
|
- Energy storage (e.g., starch, glycogen)
- Structural support (e.g., cellulose, chitin)
|
|
Typical Examples
|
glucose (aldose),
fructose (ketose),
galactose
|
sucrose (glucose + fructose), lactose (glucose + galactose), maltose (glucose + glucose)
|
starch (plant energy storage), glycogen (animal energy storage), cellulose (plant cell wall),
chitin (arthropod exoskeleton)
|
Types of Monosaccharides
Aldoses vs. Ketoses
Monosaccharides are classified into aldoses and ketoses by the position of their carbonyl group. Aldoses contain an aldehyde group (-CHO) at the terminal carbon. Examples include glucose and galactose. While ketoses have a ketone group (C=O) at the second carbon. Fructose and ribulose are known as classic ketoses. This structural difference impacts their reactivity and metabolic pathways. For instance, aldoses like glucose are central to glycolysis, while ketoses like fructose are metabolized primarily in the liver. Tests like the Seliwanoff test are used to distinguish them: ketoses react rapidly to produce a deep red color, whereas aldoses show a slower, lighter pink response.
Trioses, Tetroses, Pentoses, and Hexoses
Monosaccharides are further categorized by the number of carbon atoms:
|
Class
|
Carbon Atoms
|
Examples
|
Biological Role
|
|
Trioses
|
3
|
Glyceraldehyde, Dihydroxyacetone
|
Intermediates in glycolysis
|
|
Tetroses
|
4
|
Erythrose, Threose
|
Precursors in plant cell walls
|
|
Pentoses
|
5
|
Ribose, Xylose
|
RNA (ribose), dietary fiber (xylose)
|
|
Hexoses
|
6
|
Glucose, Fructose
|
Energy production, sweetening
|
|
Heptoses
|
7
|
Sedoheptulose
|
Rare; involved in bacterial LPS
|
Common Monosaccharides and Their Formula
Glucose is the primary fuel for cellular respiration. Its α- and β-anomers dictate how it polymerizes into starch (α-linkages) or cellulose (β-linkages). Blood glucose levels are tightly regulated by insulin and glucagon, underscoring its critical role in homeostasis. Fructose, though sweeter than glucose, is metabolized independently of insulin, making it a concern in obesity and diabetes. Galactose, when combined with glucose, forms lactose—a disaccharide crucial in infant nutrition.
|
Monosaccharide
|
Formula
|
Source
|
Function
|
|
Glucose
|
C₆H₁₂O₆
|
Fruits, vegetables
|
Primary energy source, metabolism
|
|
Fructose
|
C₆H₁₂O₆
|
Fruits, honey
|
Sweetener, metabolized in liver
|
|
Galactose
|
C₆H₁₂O₆
|
Dairy products
|
Component of lactose, cell signaling
|
|
Mannose
|
C₆H₁₂O₆
|
Plants, some fruits
|
Glycoprotein synthesis, immune modulation
|
|
Ribose
|
C₅H₁₀O₅
|
RNA, ATP
|
Genetic material, energy transfer
|
|
Deoxyribose
|
C₅H₁₀O₄
|
DNA
|
Building block of DNA, genetic material
|
|
Xylose
|
C₅H₁₀O₅
|
Wood, straw
|
Component of hemicellulose, used in industry
|
|
Arabiose
|
C₅H₁₀O₅
|
Plants, fruits
|
Part of plant polysaccharides, cell wall structure
|
|
Rhamnose
|
C₆H₁₂O₅
|
Plants, bacteria
|
Component of glycoproteins, cell wall synthesis
|
|
Allose
|
C₆H₁₂O₆
|
Synthetic
|
Rare sugar, used in research
|
|
Talose
|
C₆H₁₂O₆
|
Plants
|
Component of glycoproteins, sugar research
|
|
Idose
|
C₆H₁₂O₆
|
Found in nature
|
Used in glycosylation reactions
|
|
Muramyl Sugar
|
C₆H₁₁NO₄
|
Bacterial cell walls
|
Role in immune response, bacterial structure
|
|
Apiose
|
C₅H₈O₄
|
Plants
|
Part of plant oligosaccharides, anti-inflammatory
|
|
Xylulose
|
C₅H₁₀O₅
|
Metabolite of xylitol
|
Involved in sugar metabolism, energy production
|
|
Xylonic Acid
|
C₅H₁₀O₆
|
Plant derivatives
|
Metabolism of xylose, part of cell wall metabolism
|
Monosaccharide Analysis Methods
Modern techniques for monosaccharide identification address challenges like isomer differentiation and low abundance. HPAEC-PAD can be used to separate underivatized monosaccharides using high-pH eluents, which is ideal for analyzing plant polysaccharides. And UHPLC/QqQ-MS, widely utilized in biomarker studies, is reliable in detecting trace monosaccharides (attomolar levels) via dynamic MRM. Some derivatizations like PMP labeling enhance MS sensitivity for neutral sugars. Chiral separations need specialized columns or enzymatic assays for isolating enantiomers (e.g., D-glucose vs. L-glucose). Analyzing human milk oligosaccharides (HMOs) demands many advanced methods to resolve structurally similar sugars like fucose and sialic acid. Creative Biolabs offers a series of advanced glycan analysis technologies for monosaccharide analysis:
|
Method
|
Strengths
|
Limitations
|
|
HPLC
|
High accuracy, wide applicability
|
Requires derivatization
|
|
MS
|
Highly sensitive, ideal for complex mixtures
|
Expensive, requires high expertise
|
|
LC-ESI-MS
|
Excellent for glycoprotein profiling, sensitivity
|
High equipment cost, requires skilled operation
|
|
MALDI-TOF MS
|
Fast analysis, minimal sample preparation
|
Limited by sample size and matrix interference
|
|
HPAEC-PAD
|
Specialized for acidic sugars, high resolution
|
Requires highly pure samples
|
|
UHPLC/FLD/Q-TOF
|
High sensitivity, excellent for trace detection
|
Requires expensive equipment and specialized training
|
|
FTIR
|
Non-destructive, good for overall structure analysis
|
Low resolution for small molecules
|
|
NMR
|
Precise structural determination, non-destructive
|
Requires high concentration and specialized knowledge
|
|
TLC
|
Simple, cost-effective, and quick
|
Limited resolution for complex mixtures
|
|
Flow Cytometry
|
Enables real-time analysis, cell-specific profiling
|
Requires high cell count and specialized reagents
|
|
GC-MS
|
High sensitivity, good for volatile compounds
|
Limited to volatile glycans, requires derivatization
|
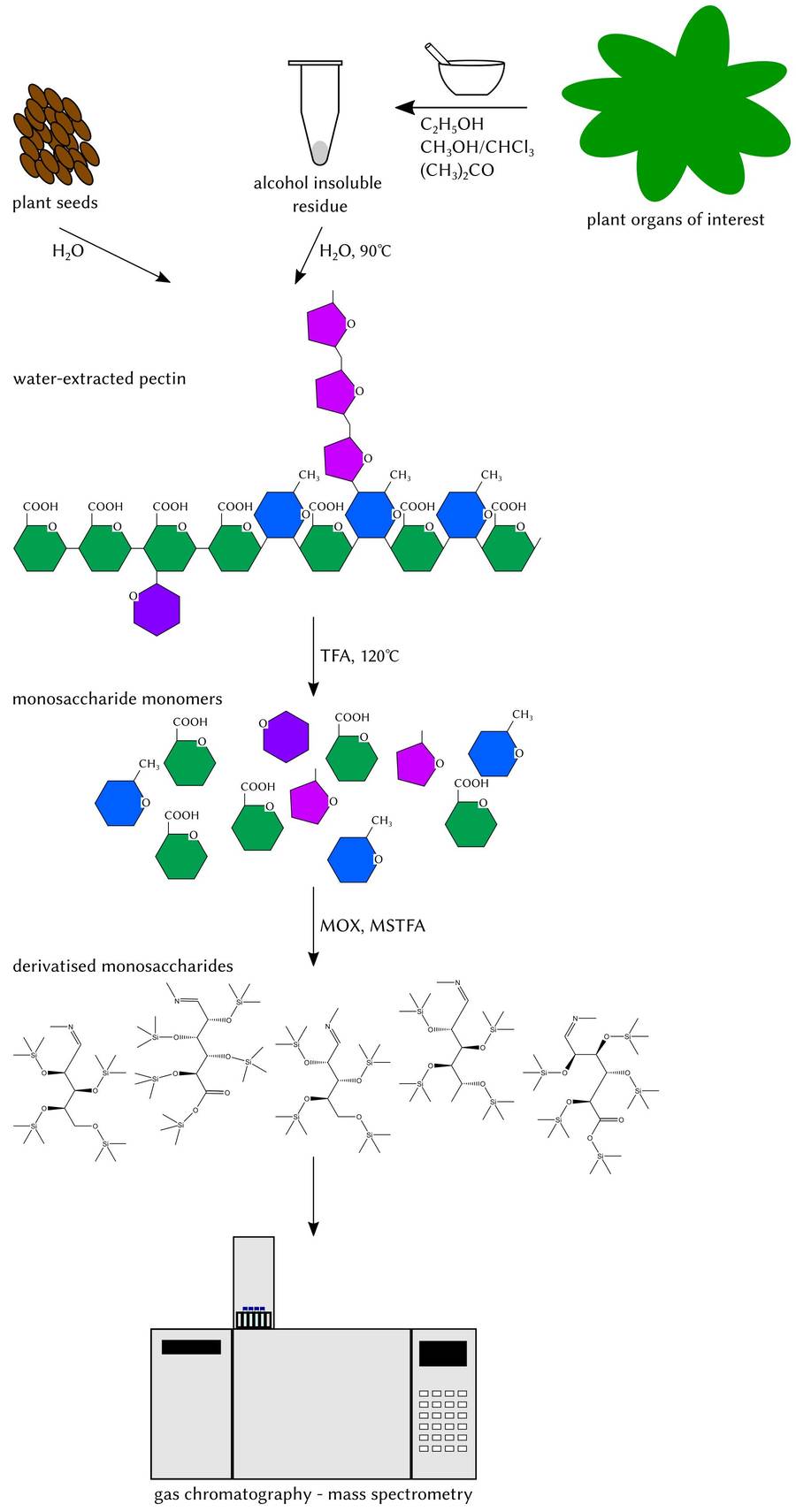 Fig.1 Monosaccharides analysis by GC-MS.1
Fig.1 Monosaccharides analysis by GC-MS.1
Applications of Monosaccharides in Biomedical Research
Drug and Vaccine Development
-
Targeted drug delivery: Mannose-coated nanoparticles exploit immune cell receptors (e.g., macrophages) for precise drug delivery.
-
Glycomimetics: Monosaccharide derivatives like zanamivir (a sialic acid analog) inhibit viral neuraminidase, treating influenza. Ethyl-α-D-furanarabinose and ethyl-β-D-fructofuranoside, two monosaccharide derivatives, have shown immunomodulatory effects by activating NF-κB pathways in macrophages, enhancing phagocytosis and cytokine secretion.
-
Glycoconjugate vaccines: Conjugating monosaccharides to carrier proteins improves antigen presentation, enabling targeted immune responses against pathogens. (Carbohydrate Chains in Vaccines)
Disease Diagnosis
-
Glycoprotein Biomarkers in Cancer: Elevated sialic acid (Neu5Gc) levels correlate with tumor progression.
-
Glycan profiling: Aberrant O-GlcNAcylation (a glucose-derived modification) signals metabolic disorders like diabetes.
-
Immune profiling: Lectin arrays functionalized with fucose or galactose detect glycan alterations on immune cells, revealing autoimmune or inflammatory states.
Cell Culture and Tissue Engineering
-
Cell media: Glucose is a staple energy source, but excess amounts can lead to oxidative stress—researchers optimize concentrations for stem cell growth.
-
Scaffold materials: Hyaluronic acid (a glucuronic acid polymer) enhances hydrogel biocompatibility in cartilage repair. (Carbohydrate Chains in Tissue Engineering)
Tailored Monosaccharides Synthesis Solutions
Creative Biolabs specializes in offering custom monosaccharide synthesis service like synthesizing rhamnose and xylose derivatives for optimizing your monosaccharide studies:
-
Glyco-engineering of antibodies to improve pharmacokinetics.
-
Designing amino monosaccharides for antimicrobial peptide conjugates.
-
Custom synthesis for rare sugars (e.g., L-rhamnose) for studying bacterial glycans.
-
Isotope-labeled sugars like 13C-glucose tracks metabolic flux in cancer cells.
-
High-throughput synthesis of oligosaccharides for vaccine development.
Advanced Monosaccharides Analytical Services
Creative Biolabs boasts a team of highly skilled analysts, dedicated to delivering comprehensive and high-quality monosaccharide analysis services with accuracy and reliability at the forefront. Beyond standard polysaccharide analysis for glucose, fructose, galactose, and mannose, we also specialize in the detection of rare monosaccharides such as apiose, xylulose, and xylonic acid. Leveraging our advanced glycosylation analysis platform, our scientists bring extensive expertise and hands-on technical experience in monosaccharide analysis, offering tailored analytical solutions to meet the specific needs of our clients.
-
Monosaccharide Profiling: Using HPAEC-PAD for acidic sugars like glucuronic acid.
-
Stability Testing: Assessing degradation of inulin (a fructose polymer) under varying pH/temperature.
If you are not sure about how to choose the best-fit method for your monosaccharides or other types of glycans, please refer to the following flowchart facilitating your decision.
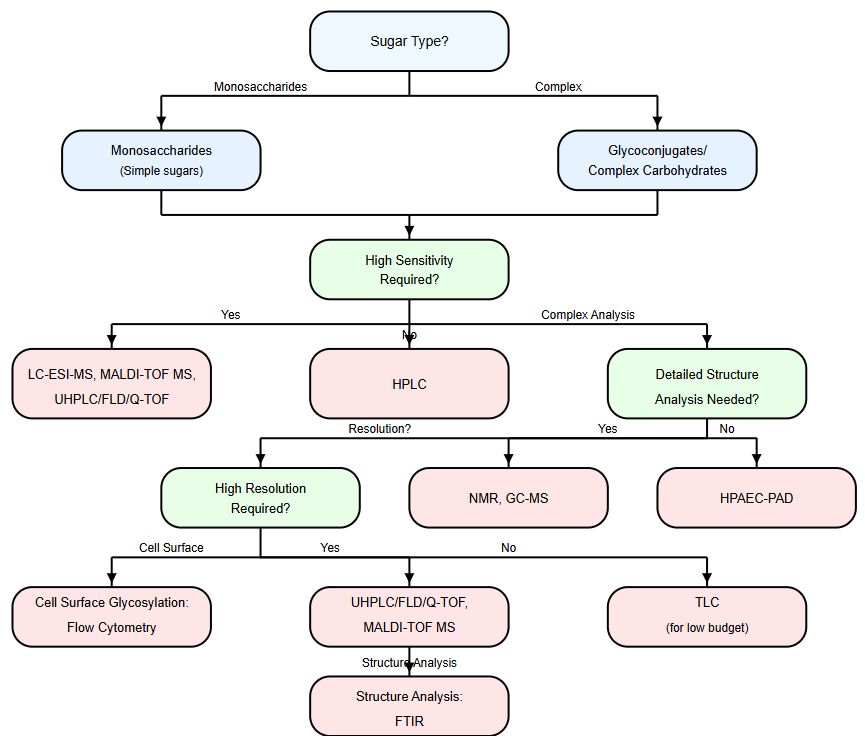 Fig.2 Glycan analysis instruction.
Fig.2 Glycan analysis instruction.
Monosaccharides are not merely energy sources but pivotal players in drug design, diagnostics, and regenerative medicine. As research uncovers their diverse roles, Creative Biolabs continues to innovate, offering bespoke synthesis and analysis services to overcome technical hurdles. For researchers seeking to explore carbohydrates in glycobiology and glycans in biological processes in depth, partnering with experts ensures precision and accelerates discovery.
Published Data
The following showcases a GC-MS chromatogram of monosaccharides derived from mucilage pectin, analyzed via the MSD ChemStation Data Analysis software. The experimental protocol commences with the cultivation of plants under controlled conditions. Subsequently, samples, such as seeds, are utilized for the extraction and hydrolysis of pectin. The resultant monosaccharides are then subjected to derivatization prior to undergoing comprehensive analysis by gas chromatography-mass spectrometry (GC-MS). During the GC-MS analysis, specific chromatographic conditions are meticulously established, including a pre-determined temperature gradient, an optimized carrier gas flow rate, and well-defined ionization parameters. These conditions ensure the accurate separation and detection of individual monosaccharides. The output chromatogram reveals distinct peaks corresponding to different monosaccharides, facilitating their precise identification based on characteristic retention times and mass spectra. The GC-MS technique stands as an indispensable analytical tool in this context. Its significance lies in its ability to concurrently detect a diverse array of monosaccharides within pectin samples. This multiplexed detection capability allows for a comprehensive and in-depth analysis of the pectin's composition. Such detailed compositional information is of utmost importance for elucidating the intricate functions of plant cell walls and understanding the underlying mechanisms of plant-pathogen interactions, thereby advancing our knowledge in the field of plant biology.
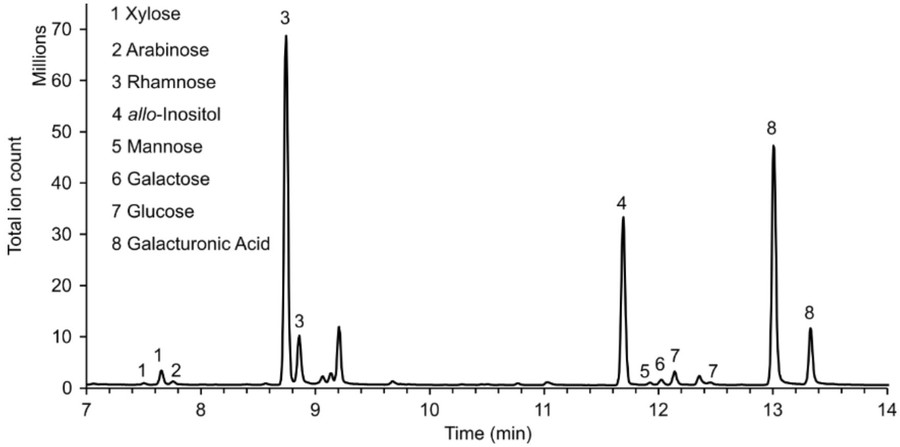 Fig.3 GC-MS analysis of mucilage pectin monosaccharides.1
Fig.3 GC-MS analysis of mucilage pectin monosaccharides.1
Reference
-
Scholz, Patricia, et al. "Analysis of Pectin-derived Monosaccharides from Arabidopsis Using GC–MS." Bio-protocol 13.16 (2023): e4746. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.21769/BioProtoc.4746
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Monosaccharides analysis by GC-MS.1
Fig.1 Monosaccharides analysis by GC-MS.1
 Fig.2 Glycan analysis instruction.
Fig.2 Glycan analysis instruction.
 Fig.3 GC-MS analysis of mucilage pectin monosaccharides.1
Fig.3 GC-MS analysis of mucilage pectin monosaccharides.1

