Introduction to Carbohydrate Chains in Biological Processes
Carbohydrate chains are fundamental to life, acting as key players in countless biological processes. Beyond providing energy, they serve as signaling molecules, structural components, and are essential for cell recognition. For example, the sialic acid residues on glycoproteins help immune cells avoid being detected, essentially allowing them to "hide" and promote immune tolerance. These carbohydrate chains also form glycoproteins and glycolipids, which are involved in everything from cell-to-cell communication to immune responses and even the spread of disease. Carbohydrates contribute to the structural stability of cell membranes, and their varied chemical properties help catalyze complex biochemical reactions—much like the glycocalyx, which coats the surface of cells, offering protection while enabling communication between them.
Why Carbohydrate Chains Are Crucial for Life at the Cellular Level
At the cellular level, carbohydrate chains are indispensable. They're at the core of how cells generate energy, like when glycogen stored in muscles and the liver is broken down to fuel cellular activity. These chains also play a structural role, being essential components of the cell membrane, where they support not only mechanical integrity but also cell recognition and signaling. Think of the ABO blood group system—specific carbohydrate chains on red blood cells determine your blood type and are responsible for the way your cells recognize each other. But their role goes beyond structure; carbohydrate chains also help proteins fold properly and get to where they're needed, guiding them through processes like cellular trafficking. High-mannose oligosaccharides, for instance, are key players in helping proteins like glycophorin fold and move within the cell, and they also help dispose of misfolded proteins through the ER-associated degradation pathway.
Carbohydrate Chains in Protein-Protein Interaction Networks
Carbohydrate chains are also integral to how proteins interact with each other. They can bind to specific parts of proteins, influencing their structure and function in ways that can't always be predicted. Many proteins are modified by glycosylation, a process that attaches carbohydrates to them, altering their behavior—for example, making them more resistant to degradation, enhancing their stability, or providing new surfaces for immune recognition. The glycosylation of the HIV-1 envelope protein gp120, for instance, helps the virus attach to and enter human cells by binding to host receptors. These carbohydrate chains also regulate how proteins interact with one another through networks of hydrogen bonds, flexible conformations, and glycosidic linkages. Carbohydrate chains in glycobiology are crucial for basic cellular signaling, disease processes like cancer metastasis, where E-cadherin glycosylation aids cell spread, and in vaccine development.
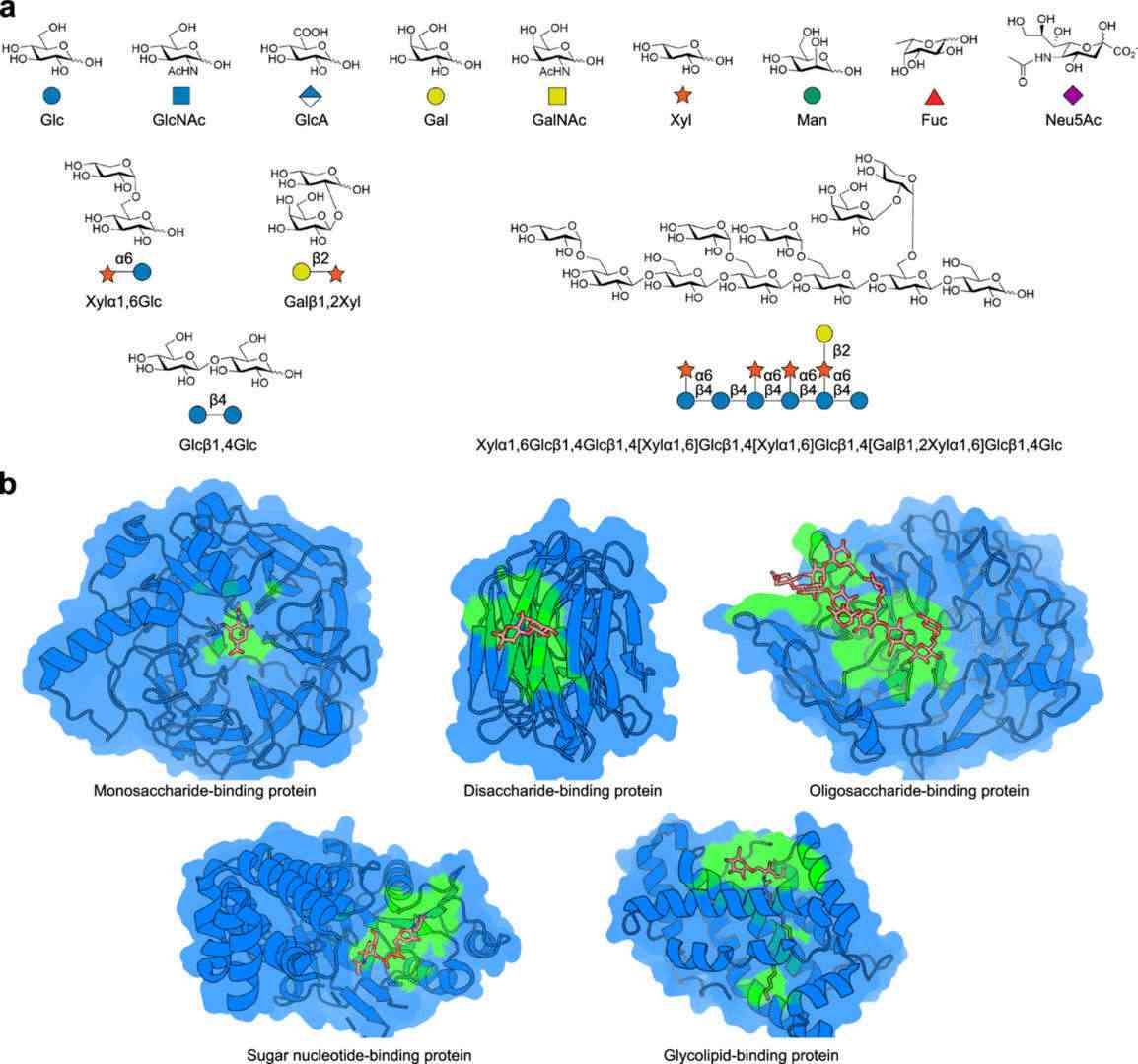 Fig.1 Variety and complexity of carbohydrates and their binding sites.1
Fig.1 Variety and complexity of carbohydrates and their binding sites.1
The Power of Carbohydrate Side Chains in Cellular Processes
Carbohydrate side chains have a powerful influence on how cells behave and interact. By binding with proteins, lipids, and other molecules, they help form complex molecular networks that regulate a range of biological functions. One key role is in signal transduction—carbohydrate side chains bind to receptors on cell surfaces, triggering pathways that control cell behavior. For instance, fucose residues on glycoproteins interact with selectins, proteins that help guide white blood cells during inflammation, highlighting the importance of carbohydrate chains in immune responses. They also play a critical role in recognizing pathogens; glycoproteins and glycolipids on cell surfaces, such as those on respiratory cells, can identify viruses like influenza and initiate an immune response. Furthermore, carbohydrate side chains influence how proteins function and where they go within the cell, using properties like hydrogen bonding and flexibility. Sialylated glycoproteins, for example, help immune cells avoid detection by the immune system, playing a crucial role in immune evasion. Ultimately, carbohydrate side chains are central to the complex biological processes that happen inside every cell. Creative Biolabs provides an in-depth understanding of the multiple roles of carbohydrate chains in biological processes, ranging from normal physiological functions to pathological changes.
Carbohydrate Sensors and Biological Recognition
Designing carbohydrate sensors is an exciting frontier in biosensor research. By developing bio-recognition elements based on carbohydrates, such as glycosylated molecules or glyconanoparticles, researchers are improving the sensitivity and selectivity of carbohydrate detection. For example, glycosylated sensors can be used for real-time monitoring of sugars like glucose and mannose, which is crucial for the diagnosis and management of diseases such as diabetes and other metabolic disorders.
The advancement of these sensors hinges on understanding the interactions between carbohydrate molecules and sensor components. This research not only focuses on achieving high sensitivity but also addresses challenges such as sensor stability, reusability, and the ability to adapt to structural changes in polysaccharides. The goal is to develop "smart" sensors that can respond dynamically to the complex variations in carbohydrate structures, offering more precise and versatile tools for medical diagnostics and research. To better understand advancements in carbohydrate sensors, the table below compares different types based on their target sugars, applications, and key advantages and limitations. At Creative Biolabs, we have developed advanced platforms for custom carbohydrate synthesis and carbohydrate analysis services, suitable for your carbohydrate sensors development and other carbohydrate-related research.
|
Sensor Type
|
Target Sugars
|
Application Areas
|
Advantages
|
Disadvantages
|
Fluorescent Sensor
(Fluorescence Labeling)
|
Glucose, Mannose
|
Diabetes diagnosis, Metabolic monitoring
|
High sensitivity, Real-time monitoring
|
Stability issues, Limited lifespan
|
|
Ionic Liquid Sensor
|
Glucose, Fructose
|
Clinical diagnostics, Food monitoring
|
High selectivity, Strong adaptability
|
Higher cost, Complex design
|
|
Glyconanoparticle Sensor
|
Glucose, Lactose
|
Biomedical research, Drug development
|
High stability, Reusable
|
Slower response time
|
|
Enzyme-based Sensor
|
Glucose, Galactose
|
Glucose level monitoring in clinical settings
|
High specificity, Direct response
|
Limited to certain sugars
|
Development of Bio-Mimetic Receptors
In recent years, the field of carbohydrate recognition has seen remarkable advancements, largely driven by the increasing understanding of how sugar molecules interact with biomolecules. Bio-mimetic receptors, designed to imitate natural receptors, have become a key area of focus. These artificial receptors are inspired by the way sugars and biomolecules communicate in nature, but with a twist: they are engineered to have superior selectivity and affinity. A study published in December 2024 introduced a novel covalent cage receptor with an impressive affinity for glucose in biological fluids. This breakthrough not only offers a promising step forward in glucose detection and binding but also holds significant potential in real-time monitoring of diseases like diabetes. Beyond foundational research, bio-mimetic receptors are now being applied in biosensors, drug screening, and vaccine development, offering exciting new opportunities in these fields.
Molecular Recognition Mechanisms
Molecular recognition is the precise interaction between a biomolecule and its target, a process that is fundamental in areas such as disease diagnosis, drug design, and therapy. In carbohydrate recognition, non-covalent interactions—such as hydrogen bonds, hydrophobic effects, and electrostatic forces—play a pivotal role in ensuring specificity. A 2021 study demonstrated the use of a vibration-induced emission fluorescence chemical sensor to selectively recognize glucose. This technology alters the environment around carbohydrate molecules, changing their fluorescence characteristics and thus enabling the detection of glucose presence and concentration. This research highlights the power of non-covalent interactions in sensor design and opens the door to more sensitive carbohydrate sensors.
Polysaccharide Recognition
Polysaccharides are complex sugar molecules that play critical roles in a wide array of biological processes. In 2016, researchers developed a new linear receptor specifically designed for recognizing polysaccharides. The structure of this receptor allows it to effectively interact with polysaccharide chains, providing a valuable tool for the study of their recognition and functionality. This receptor not only aids in understanding the biological roles of polysaccharides but also holds promise for the development of carbohydrate-based drugs, vaccines, and therapeutic strategies targeting polysaccharide-related diseases. Additionally, polysaccharide recognition is closely tied to the development of glycovaccines—vaccines that mimic the sugar structures of pathogens to induce immune responses, improving vaccine efficacy.
Reference
-
He, Xinheng, et al. "Highly accurate carbohydrate-binding site prediction with DeepGlycanSite." Nature Communications 15.1 (2024): 5163. Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Variety and complexity of carbohydrates and their binding sites.1
Fig.1 Variety and complexity of carbohydrates and their binding sites.1

