The Importance of Glycans in Ocular Tissue Health
The human eye is one of the most intricate organs in the body, and understanding the molecular components that help maintain its functionality is crucial for tackling eye diseases. One such component, often overlooked, is carbohydrate chains (glycans) chains attached to proteins and lipids. These glycans play an essential role in maintaining the delicate balance of ocular health by influencing cellular processes such as signaling, adhesion, and immune defense. Specifically, the glycosylation of proteins on the ocular surface is a fundamental process in ensuring the stability and health of the cornea and retina. The ocular surface is lined with a glycocalyx, a carbohydrate-rich structure that forms a protective barrier. This glycocalyx is composed of glycoproteins, such as mucins, which play an essential role in protecting the eye from external stressors. Mucin-type O-glycans are of particular importance, as they provide hydration, lubrication, and pathogen resistance. These carbohydrate structures are highly dynamic, and their presence or absence can significantly impact ocular tissue health, especially in diseases like dry eye disease (DED), macular degeneration, and diabetic retinopathy.
At Creative Biolabs, we understand the vital role of glycans in ocular health. Our carbohydrate analysis services are designed to help researchers explore the complex carbohydrate profiles present on ocular tissues. We provide advanced platforms to assist in the identification and analysis of glycan structures. These services can be crucial for understanding how glycosylation influences retinal health and function.
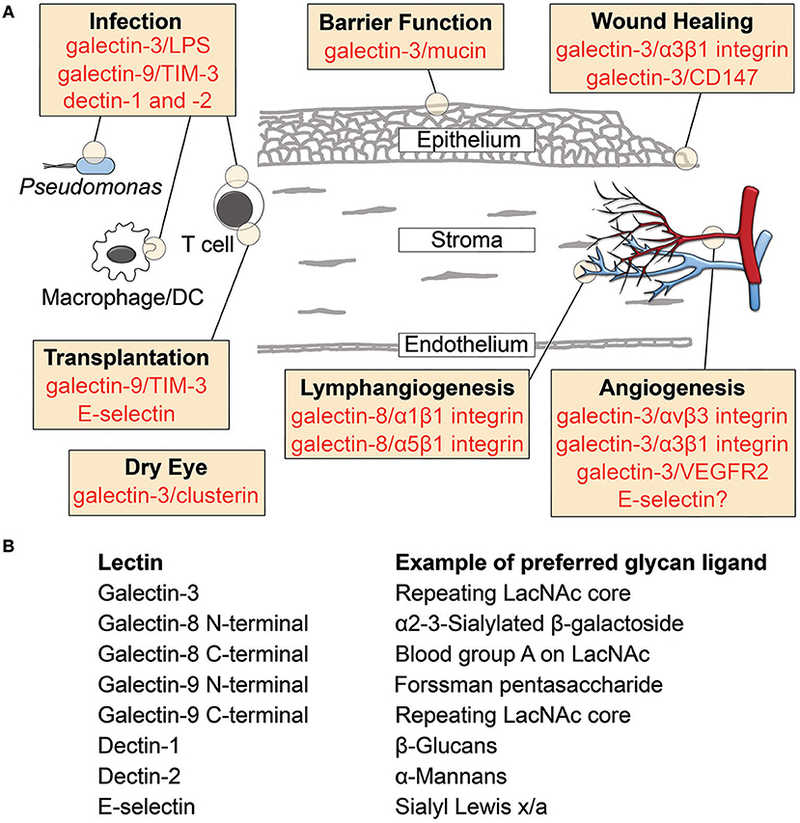 Fig.1 Lectin-glycan interactions in cornea.1
Fig.1 Lectin-glycan interactions in cornea.1
Glycans and Glycosylation in Retinal Function
Glycans maintain essential functions both on the ocular surface and within retinal operations. The retina serves as a specialized tissue that transforms light into electrical impulses which the brain deciphers as visual information. Precise glycosylation patterns enable retinal cells to maintain their structure and function while ensuring proper interactions with neighboring cells. Glycosylation patterns must be precise for retinal neurons, endothelial cells and glial cells to maintain their function. Any disturbance in the glycosylation process of retinal cells will result in retinal degeneration which impairs vision. Protein glycosylation at the retinal surface functions to support cell-cell communication while contributing to extracellular matrix stability. Glycans play multiple roles in retinal health by protecting against oxidative damage and sustaining neuronal survival. Disrupted glycosylation can caused diseases like:
-
Retinitis Pigmentosa (RP): This genetic disorder is characterized by the progressive degeneration of retinal photoreceptor cells. Disruptions in glycosylation affect the cell membrane stability and functionality of photoreceptors, accelerating their degeneration.
-
Age-Related Macular Degeneration (AMD): Abnormal glycosylation of retinal cells, particularly in the retinal pigment epithelium (RPE), contributes to the breakdown of the blood-retinal barrier and the accumulation of waste products, leading to damage in the macula and central vision loss.
-
Diabetic Retinopathy: In diabetes, altered glycosylation of proteins in retinal endothelial cells affects the stability of blood vessels, contributing to vascular leakage, microaneurysm formation, and retinal edema. This leads to poor oxygen supply to the retina and subsequent damage.
-
Leber Congenital Amaurosis (LCA): A rare genetic disorder where mutations in glycosylation pathways contribute to the loss of photoreceptor function and retinal degeneration from an early age.
Carbohydrate-Modified Proteins in Retinal Diseases
The retina relies heavily on carbohydrate-modified proteins, particularly glycoproteins, for maintaining its structure and function. These modifications are crucial for protein folding, stability, and cellular interactions. However, in retinal diseases, the glycosylation of proteins is often altered, leading to disease progression. One of the key aspects of retinal disease development is the alteration of glycoproteins involved in cellular signaling and adhesion. In diseases such as diabetic retinopathy and macular degeneration, abnormalities in the glycosylation patterns of glycoproteins impair the retina's ability to function properly. The alteration of glycans in retinal function can also lead to improper cell signaling, which may activate pathways that contribute to retinal cell death. This phenomenon is especially pronounced in diseases like retinitis pigmentosa, where glycosylation defects accelerate the degeneration of photoreceptor cells in the retina. Here is a table that lists some glycoproteins involved in retinal disease development, with examples of their roles in retinal dysfunction:
|
Glycoprotein
|
Role in Retinal Disease Development
|
Associated Diseases
|
|
MUC16
|
Protects the ocular surface from infection and maintains cell adhesion. In retinal diseases, its glycosylation is altered, leading to compromised cell protection and increased inflammation.
|
Age-related macular degeneration (AMD), Diabetic retinopathy
|
|
Galectin-3
|
A carbohydrate-binding protein that interacts with glycoproteins like MUC16, maintaining barrier function. In retinal diseases, altered galectin-3 binding leads to tissue damage.
|
Retinitis pigmentosa, Diabetic retinopathy
|
|
Fibronectin
|
Plays a key role in retinal cell adhesion and migration. Abnormal fibronectin expression is seen in conditions like retinal fibrosis, which leads to scarring.
|
Retinal fibrosis, Diabetic retinopathy
|
|
Laminin
|
Essential for the structural integrity of the retinal extracellular matrix. Glycosylation defects can result in the breakdown of the retinal architecture and affect cell signaling.
|
Retinitis pigmentosa, Retinal detachment
|
|
CD44
|
A cell surface glycoprotein involved in cell adhesion and migration. Altered CD44 glycosylation is linked to increased retinal cell migration and fibrosis.
|
Diabetic retinopathy, Retinal neovascularization
|
|
TGF-β receptors
|
Involved in retinal cell growth, differentiation, and immune response. Glycosylation changes can alter TGF-β signaling, leading to retinal cell apoptosis and fibrosis.
|
Diabetic retinopathy, Age-related macular degeneration (AMD)
|
To support research into these important glycoproteins, Creative Biolabs offers services such as Custom Glycoprotein Synthesis Services, Glycoprotein Analysis Services and Glycoprotein Quantification. These services help researchers synthesize glycoproteins and analyze the structural and functional changes in glycoproteins that occur in retinal diseases, aiding in the development of more effective therapeutic strategies.
Stem Cell Research for Vision: A New Frontier for Retinal Repair
One notable example of stem cell therapy in retinal regeneration comes from a groundbreaking study where induced pluripotent stem cells (iPSCs) were used to treat age-related macular degeneration (AMD). In this study, researchers derived iPSCs from patients' own retinal cells and successfully differentiated them into retinal pigment epithelial (RPE) cells. These RPE cells were then transplanted back into animal models. The iPSCs were genetically corrected using CRISPR-based technologies, targeting specific genetic mutations that cause AMD. The resulting corrected RPE cells integrated into the retina and restored some functionality, demonstrating that iPSCs can not only regenerate retinal tissue but also potentially correct underlying genetic defects in retinal diseases. This experiment provides important insights into the feasibility of personalized stem cell therapies for treating genetically inherited retinal conditions.
Another promising example comes from research using human embryonic stem cells (hESCs) to generate retinal neurons for treating retinitis pigmentosa. In this experiment, hESCs were differentiated into retinal progenitor cells (RPCs), which were then transplanted into animal models. These RPCs integrated into the retina and differentiated into photoreceptor cells, improving vision in the animals. The study utilized optogenetics, a technique that enables real-time control of light-sensitive cells, to monitor the integration of the transplanted retinal cells. The results showed that these hESC-derived cells could restore some functional retinal activity, providing significant evidence that stem cell-based therapies hold potential for not just regenerating retinal tissue but also restoring functional vision. This research opens doors to potential clinical applications for patients with retinal degenerative diseases.
At Creative Biolabs, we provide advanced services such as therapeutic glycoprotein development services and genetic glycoengineering to support stem cell research in retinal repair. By incorporating glycoengineering strategies into stem cell therapies, we help researchers enhance the functionality and integration of stem cell-derived retinal cells.
The integration of glycosylation research and stem cell therapy represents a cutting-edge approach for treating retinal diseases. Understanding the role of glycans in stem cell differentiation and integration into the retinal tissue is crucial for improving the success of these therapies. By modulating glycosylation pathways, researchers are working to enhance the regenerative potential of stem cells and improve their ability to repair damaged retinal tissue. At Creative Biolabs, we are committed to advancing research in both glycosylation and stem cell therapy to provide cutting-edge solutions for ocular health. Our glycosylation site mapping and glycan profiling services help researchers gain a deeper understanding of how glycosylation affects stem cell behavior. These insights are crucial for developing more effective stem cell-based treatments for retinal diseases, offering hope for millions of people affected by retinal degeneration.
Reference
-
AbuSamra, Dina B., and Pablo Argüeso. "Lectin-glycan interactions in corneal infection and inflammation." Frontiers in Immunology 9 (2018): 2338. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fimmu.2018.02338
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Lectin-glycan interactions in cornea.1
Fig.1 Lectin-glycan interactions in cornea.1

