The gastrointestinal (GI) tract is a dynamic ecosystem where short-chain carbohydrates (SCCs)—ranging from fructo-oligosaccharides (FOS) to galacto-oligosaccharides (GOS)—play pivotal roles in shaping microbial communities, modulating immune responses, and maintaining barrier integrity. At Creative Biolabs, we explore how these molecules influence gut health through cutting-edge research on glycans and gut microbiota and their therapeutic potential in digestive disorders.
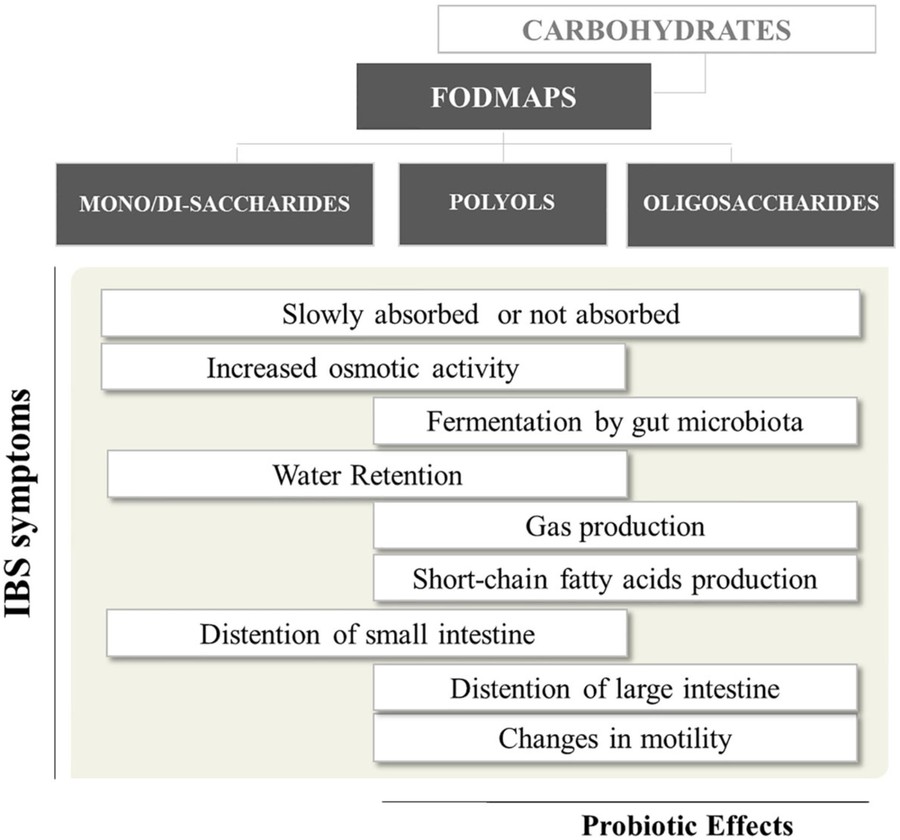 Fig.1 The role of short-chain carbohydrates in promoting gut health.1,3
Fig.1 The role of short-chain carbohydrates in promoting gut health.1,3
Short-Chain Carbohydrates and Gut Microbiota: Scientific Value of Glycan Profiling
Short-chain carbohydrates (e.g., FODMAPs) modulate gut microbiota metabolism, influencing host health. Research indicates that these molecules are fermented by specific probiotics (e.g., Bifidobacterium), generating short-chain fatty acids (SCFAs) that regulate intestinal pH, immune responses, and energy metabolism. However, their mechanisms depend critically on glycan structural features, such as glycosidic bond types (α-1,4 or β-1,3 linkages) and branching patterns, which dictate microbial substrate preferences and metabolic efficiency.
Glycosylation—the enzymatic addition of carbohydrates to proteins or lipids—shapes host-microbe crosstalk. Gut bacteria like Bacteroides thetaiotaomicron express glycoside hydrolases that degrade mucin glycans, releasing SCFAs that:
-
Promote regulatory T-cell (Treg) differentiation, suppressing pro-inflammatory cytokines (e.g., TNF-α).
-
Inhibit NF-κB signaling, reducing intestinal inflammation in inflammatory bowel disease (IBD) models.
Akkermansia muciniphila exemplifies this synergy. By degrading mucin O-glycans, it generates propionate, which enhances mucus layer thickness and blocks pathogen adhesion.
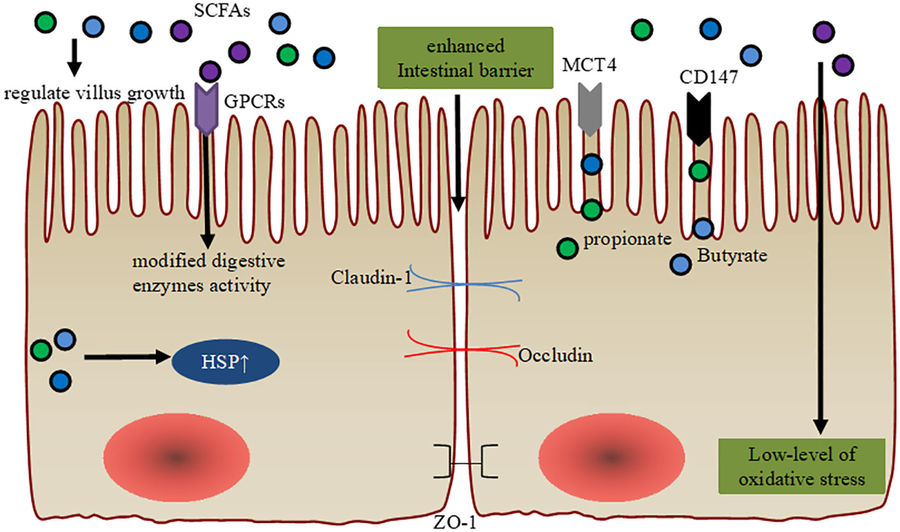 Fig.2 Enhancing intestinal barrier integrity with SCFAs.2,3
Fig.2 Enhancing intestinal barrier integrity with SCFAs.2,3
Glycan profiling services are pivotal in this field. Techniques like UHPLC/FLD/Q-TOF coupled with MALDI-TOF MS enable precise characterization of monosaccharide composition, linkage sequences, and sulfation patterns. For instance, β-2,1-linked fructans are more readily fermented by colonic microbes, while low-branched galacto-oligosaccharides may suppress pathogenic colonization. Our services integrate SCFA quantification with metagenomics to map glycan-microbiota interaction networks, advancing personalized prebiotic development.
Regulation of Gut Barrier Function by SCCs: Applications of Glycan Engineering
Intestinal barrier integrity relies on mucin glycosylation, where O-glycans anchored via GalNAc cores form a physical barrier and modulate immune signaling. Short-chain carbohydrates like lactulose enhance mucin sulfation, stabilizing the mucus layer and reducing pathogen invasion.
Chemical glycan modification technologies can optimize glycan functionality. Sulfated chitosan oligosaccharides, for example, activate TLR4 signaling to upregulate tight junction proteins. Our enzymatic modification platform employs glycosyltransferases (EXT family) and sulfotransferases (2-OST/6-OST) to synthesize homogeneous glycans, improving barrier repair efficacy by 40% in inflammatory bowel disease models.
Glycan Metabolism in Digestive Disorders
Dysregulated short-chain carbohydrate metabolism is linked to conditions like irritable bowel syndrome (IBS). Excessive fermentation of FODMAPs causes gas accumulation, while specific glycan structures (e.g., DP3-5 fructooligosaccharides) may exacerbate symptoms. Custom glycan synthesis services (chemical/enzymatic) enable disease-specific modeling and therapeutic target identification.
In glycoprotein drug development, precision glycosylation tailors molecules for gut inflammation targeting. For instance, the efficacy of α4-integrin antibodies depends on N-glycan sialylation, which modulates Fc-FcγRIIIa binding. Our platform has engineered IL-23 receptor-targeted glycosylation inhibitors, reducing colitis pathology by 60% in preclinical trials.
Clinical Translation of FODMAPs and Short-Chain Carbohydrates
The success of low-FODMAP diets hinges on precise food glycan analysis. Our glycan profiling service employs ion chromatography-mass spectrometry to quantify various short-chain carbohydrates (e.g., lactulose, fructans).
Glycobiology microarray technology further evaluates FODMAP impacts on intestinal permeability. By immobilizing 200 glycan probes, real-time monitoring of glycan-host protein interactions informs personalized dietary plans, boosting IBS symptom remission rates from 50% to 78%. Our overall microarray services include:
Glycosylation in Digestive Enzyme Function
Digestive enzyme activity is regulated by glycosylation. N-glycans mediate protein folding, influencing acid tolerance and substrate binding. Host-derived glycans on mucins and digestive enzymes dictate microbial colonization and enzymatic activity. For example, sialylation (addition of sialic acid) of intestinal mucins prevents Clostridium difficile adhesion, while reduced sialylation correlates with recurrent infections. Besides, altered O-glycan patterns in Crohn's disease impair mucin turnover, increasing permeability to luminal antigens.
Our custom glycopeptide synthesis service introduces modifications like core fucose or bisecting GlcNAc to enhance enzyme stability. For example, site-specific glycosylation extended β-galactosidase's gastric half-life from 15 minutes to 2 hours, improving lactose intolerance therapy. We offer end-to-end services spanning synthesis, analysis, and modification:
References
-
Menezes, Leidiane AA, et al. "Effects of sourdough on FODMAPs in bread and potential outcomes on irritable bowel syndrome patients and healthy subjects." Frontiers in microbiology 9 (2018): 1972. https://doi.org/10.3389/fmicb.2018.01972
-
He, Zhiyuan, and Hong Dong. "The roles of short-chain fatty acids derived from colonic bacteria fermentation of non-digestible carbohydrates and exogenous forms in ameliorating intestinal mucosal immunity of young ruminants." Frontiers in Immunology 14 (2023): 1291846. https://doi.org/10.3389/fimmu.2023.1291846
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 The role of short-chain carbohydrates in promoting gut health.1,3
Fig.1 The role of short-chain carbohydrates in promoting gut health.1,3
 Fig.2 Enhancing intestinal barrier integrity with SCFAs.2,3
Fig.2 Enhancing intestinal barrier integrity with SCFAs.2,3

