Carbohydrate Chains in Immune Recognition
Carbohydrate chains, like the "cryptic keys" for the precise operation of the immune system, play an indispensable role in immune defense and profoundly influence several key processes. As receptor-binding sites, they are just like the "bridges" for the interaction between immune cells and other molecules, being crucial for the interactions among immune cells and other molecules. On the cell surface, there are various special carbohydrate structures, such as lectins and selectins. They are like loyal "guards", tightly binding to the receptors and shouldering the important tasks of triggering immune responses and regulating cell signaling. Glycans also play a pivotal role in the communication of immune cells. They are involved in the adhesion and movement of immune cells, just as if they provide a "navigation map" for immune cells, helping immune cells communicate with each other and guiding them to the sites of infection or inflammation. The alteration of glycan structures on immune cells, like changing the "battle commands" of the cells, can affect their functions and responses to pathogens.
In terms of pathogen recognition, carbohydrate chains are a crucial part of immune defense. Many pathogens exhibit unique carbohydrate patterns. These patterns are like the "flags of invasion" and will be recognized as foreign by immune receptors, thus triggering immune responses such as phagocytosis or antibody production. At Creative Biolabs, we specialize in providing advanced carbohydrate-related research services, covering carbohydrate analysis services, receptor - ligand binding assays, and immune system profiling. Whether you are exploring the role of carbohydrates in immune modulation or researching pathogen recognition mechanisms, we can tailor - make solutions for you, propel your research forward, deepen your understanding of the interactions within the immune system, and let us jointly explore this mysterious immune field.
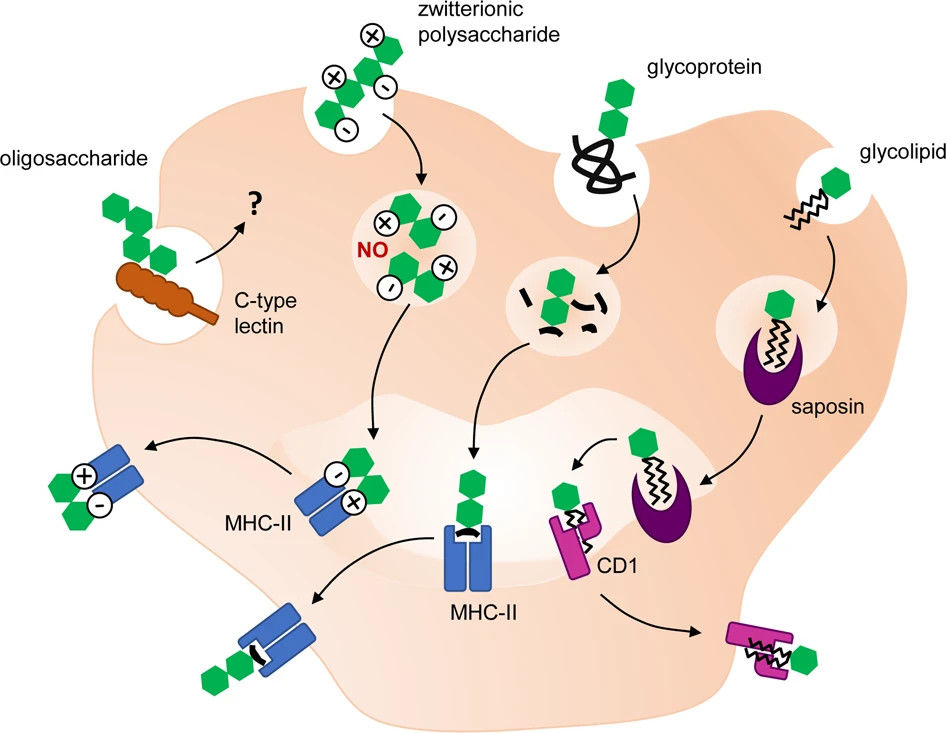 Fig.1 Antigen presentation of carbohydrate structures.1
Fig.1 Antigen presentation of carbohydrate structures.1
Carbohydrates as Receptor Binding Sites
Carbohydrate chains serve as the biological "ID cards" that enable immune systems to distinguish friend from foe. These complex sugar structures, covalently attached to proteins or lipids (glycoconjugates), are ubiquitous on cell surfaces and pathogen envelopes. Their diversity arises from monosaccharide composition, linkage patterns, and branching—a "glycocode" that immune receptors evolutionarily learn to decipher. For example, the α-2,3 vs. α-2,6 sialic acid linkage specificity of influenza hemagglutinin (HA) directly correlates with zoonotic transmission risks. Avian influenza viruses bind α-2,3-linked sialic acids dominant in avian intestinal epithelia, while human-adapted strains target α-2,6-linked forms in human respiratory tracts. This host specificity is further modulated by glycan density: high sialic acid concentrations enhance viral attachment but may sterically hinder HA conformational changes required for membrane fusion. Such glycan-receptor interplay explains why zanamivir, a sialic acid analog, effectively blocks HA-glycan interactions across multiple influenza subtypes. Creative Biolabs provides glycan-receptor interaction analysis services to study carbohydrate chains in immune response and accelerate drug discovery.
Glycans in Immune Cell Communication
Beyond pathogen recognition, carbohydrate chains orchestrate immune cell crosstalk through dynamic "sugar codes." Dendritic cells (DCs) deploy C-type lectin receptors (CLRs) like DC-SIGN to scan pathogen mannose residues—a signature absent in human high-mannose glycans. Binding triggers divergent signaling: high-affinity mannose recognition activates NF-κB for pro-inflammatory responses, whereas low-affinity interactions induce IL-10-mediated tolerance. Carbohydrate chains like ICAM-3 further mediate immune synapse formation, bridging innate and adaptive immunity. Recent advances in glycopolymer engineering exploit this mechanism. For instance, mannose-fucose copolymers redirect DC polarization toward anti-tumor Th1 responses, achieving 60% tumor regression in murine melanoma models. We provide advanced glycopolymer platform to design therapies targeting carbohydrate chains in immune system recognition.
Carbohydrate Chains in Pathogen Recognition
The immune system discriminates "self" from "non-self" through missing/altered self-recognition. The immune system's ability to detect invaders hinges on conserved carbohydrate patterns. Macrophage mannose receptors (MMR) recognize terminal mannose or fucose on bacterial glycans, activating Syk kinase and NLRP3 inflammasomes. Fungal β-1,3-glucan binds Dectin-1, initiating Syk-CARD9 signaling to produce IL-1β and IL-23—a pathway critical for antifungal defense. Meanwhile, Galectin-3 assembles β-galactoside lattices on microbial surfaces, amplifying TLR4 responses by 10-fold. Notably, MHC-II presents glycopeptide antigens via the "CLIP-swap" mechanism, where invariant chain-derived peptides are replaced by pathogen-derived glycopeptides—a process requiring T cell receptor cross-reactivity with glycan-modified epitopes.
Carbohydrate Chains in Immune Evasion
Pathogens exploit carbohydrate chains as molecular camouflage. E. coli O86:B7 expresses α-Gal LPS mimicking human blood group B antigens, evading 5% of the population lacking anti-α-Gal IgM. HIV epitomizes glycan shielding: its gp120 envelope protein is 50% covered by N-glycans, creating a moving "glycan fence" that blocks antibody access. The broadly neutralizing antibody 2G12 circumvents this by binding a unique mannose patch—a feat undermined by rapid glycosylation site mutations. Similarly, Trypanosoma cruzi masks its surface with sialylated mucins hijacked from host cells, reducing complement activation by 70%. Utilize our glycan profiling service, glycomic profiling service, glycosylation site mapping service, and glycan sequencing service to counter carbohydrate chains in immune evasion.
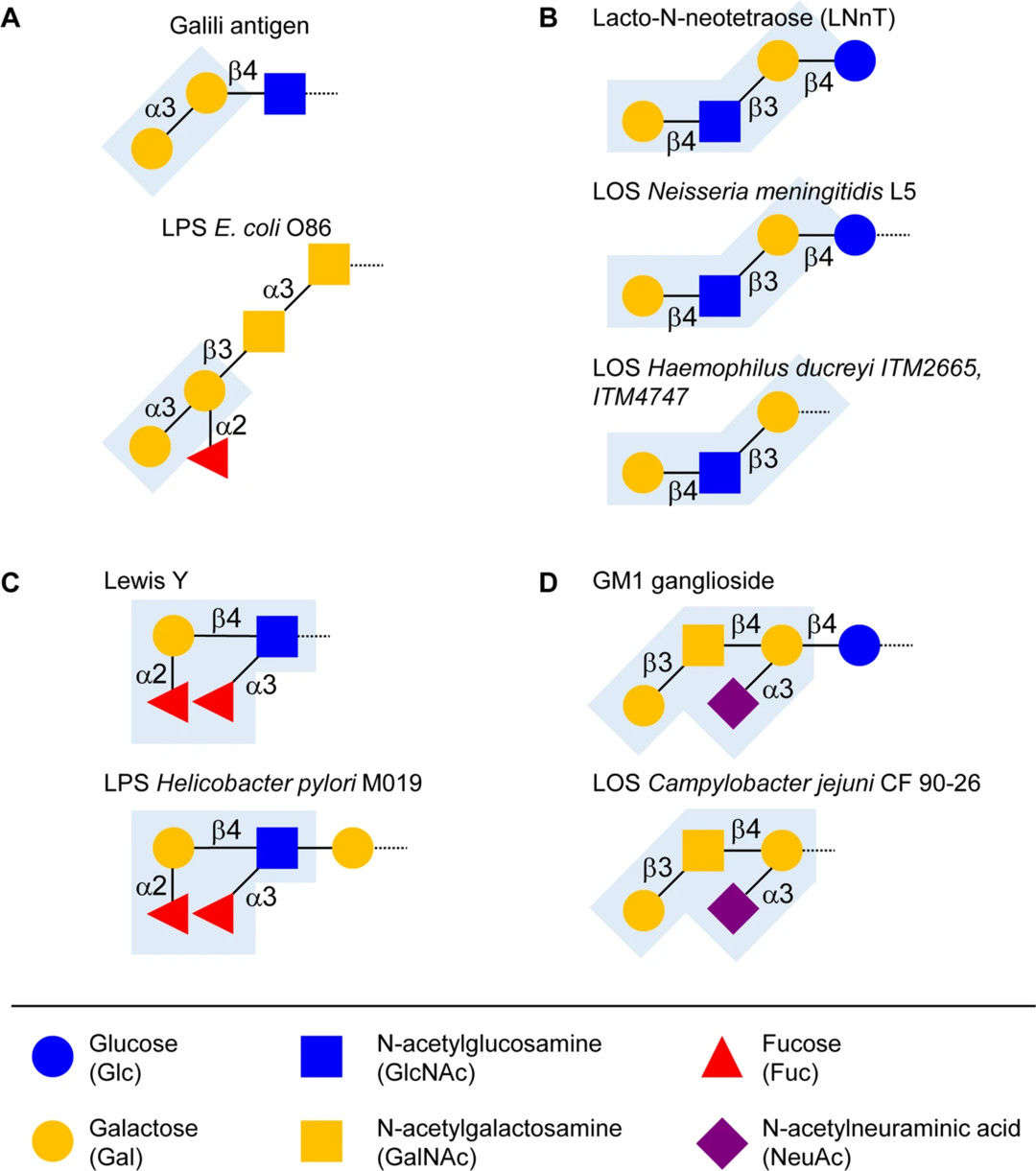 Fig.2 Molecular mimicry between animal/bacterial glycans.1
Fig.2 Molecular mimicry between animal/bacterial glycans.1
Carbohydrate Chains as Diagnostic Biomarkers
Aberrant glycosylation is a hallmark of disease, offering non-invasive diagnostic opportunities. In inflammatory bowel disease (IBD), reduced mucin O-glycan sulfonation disrupts gut barrier integrity, while elevated anti-laminaribioside antibodies distinguish Crohn's from ulcerative colitis. Machine learning-enhanced glycomics now achieves 89% accuracy in differentiating intestinal tuberculosis from Crohn's using serum N-glycan profiles. Guillain-Barré syndrome diagnosis leverages glycan microarrays to detect anti-GM1 antibodies—a 95% specific biomarker. Emerging techniques like ion mobility-MS further resolve isomeric glycans, identifying tumor-specific sialyl-Tn antigens with 10 ppm mass accuracy. Creative Biolabs provides multiple glycobiology microarray platform to exploit carbohydrate chains in immune system recognition for precision diagnostics.
Engineering Carbohydrate Side Chain Antibodies
Antibody glycoengineering is revolutionizing immunotherapy. Defucosylation of Fc N-glycans (e.g., Mogamulizumab) enhances FcγRIIIa binding affinity by 100-fold, boosting NK-mediated ADCC against lymphoma cells. Sialylation of IgG Fc domains extends serum half-life to 45 days via ASGPR-mediated recycling—a strategy employed by Ravulizumab for paroxysmal nocturnal hemoglobinuria. Bispecific antibodies now combine tumor-associated carbohydrate chains (e.g., Tn antigen) with PD-1 blockade, achieving dual targeting in solid tumors. Gene-edited CHO cells with knocked-out FUT8 genes produce afucosylated antibodies with batch-to-batch glycan consistency <2% CV. If you are interested in carbohydrate-based antibody engineering, come and partner with us to optimize carbohydrate chains and antibody binding for your biologics pipeline.
Reference
-
Kappler, Katharina, and Thierry Hennet. "Emergence and significance of carbohydrate-specific antibodies." Genes & Immunity 21.4 (2020): 224-239. Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Antigen presentation of carbohydrate structures.1
Fig.1 Antigen presentation of carbohydrate structures.1
 Fig.2 Molecular mimicry between animal/bacterial glycans.1
Fig.2 Molecular mimicry between animal/bacterial glycans.1

