As vital elements of human blood essential for multiple physiological processes blood cells participate actively in many bodily functions. These carbohydrate chains serve as crucial molecular components that sustain cell integrity, promote immune responses, and facilitate cellular interactions.
The Basic Composition of Carbohydrate Chains Found in Blood Cells
Glycans, which are carbohydrate chains comprise complex molecules consisting of monosaccharides connected by glycosidic bonds. These glycan structures play essential roles in blood cell biology, influencing functions such as immune recognition and cell adhesion in both red and white blood cells.
Monosaccharide Building Blocks
Blood cell carbohydrate chains are made up of monosaccharides including glucose (Glc), galactose (Gal), and mannose (Man). The ABO blood type antigens present on erythrocyte surfaces are the result of distinct monosaccharide structures. The A antigen features N-acetylgalactosamine (GalNAc) connected to the sugar backbone whereas the B antigen has galactose directly attached to the chain. The variations in blood group types arise from subtle changes in glycosylation processes.
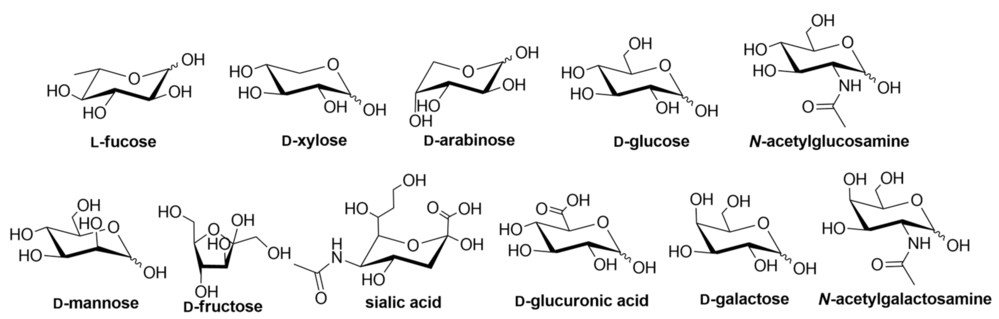 Fig.1 Structural variations of carbohydrate building blocks in nature.1,3
Fig.1 Structural variations of carbohydrate building blocks in nature.1,3
Linkage Types
The structure and function of carbohydrate chains depend on the types of linkages present. Monosaccharides are commonly connected through alpha (α)-glycosidic bonds and beta (β)-glycosidic bonds. For example, the disaccharide lactose consists of galactose and glucose molecules linked by a β-1,4 glycosidic bond. These various glycan linkage types create structural diversity in the glycans found on the surfaces of erythrocytes and leukocytes, which in turn impact functional properties such as antigen detection and immune cell adhesion.
Our Services: Glycan Structure Analysis
Creative Biolabs offers advanced glycan analysis using mass spectrometry and chromatography to accurately determine monosaccharide composition and linkage types in blood cell carbohydrate chains. Our reliable data services support both basic research and drug development objectives.
Biosynthesis of Carbohydrate Chains in Blood Cells
The production of carbohydrate chains within blood cells is tightly regulated by multiple enzymes including glycosyltransferases and fucosyltransferases which attach sugar groups to proteins and lipids. The glycosylation process in red blood cells depends on specific enzymes including α-1,3-galactosyltransferase, which is crucial for creating A or B blood type antigens. Changes in enzyme activity levels can alter the expression of surface antigens on red blood cells, affecting their functional characteristics. In leukocytes, the production of carbohydrate chains has unique features due to their specialized immune functions. Fucosyltransferases in neutrophils build glycan structures that enable cell adhesion and help immune cells travel to infection or inflammatory sites. In contrast, these enzymes function differently in erythrocytes, where they play a minimal role in cell adhesion.
Our Services: Enzyme Activity Testing
Creative Biolabs provides extensive glyan-related enzyme activity analysis services for enzymes that play critical roles in blood cell glycosylation. Our testing services examine glycosyltransferases and fucosyltransferases to reveal detailed information about the enzymatic processes that produce carbohydrate chains.
Function of Carbohydrate Chains in Routine Blood Cell Activities
Carbohydrate chains are essential for everyday functions of blood cells. The proper operation of blood cells depends on the presence of carbohydrate chains. These chains help preserve red blood cell structure and enable leukocyte movement during immune responses. Erythrocyte surface carbohydrate chains contribute to structural stability, allowing red blood cells to withstand the mechanical pressures encountered while flowing through the bloodstream. In the case of sickle cell anemia, mutations in hemoglobin alter surface glycosylation, which impairs red blood cells' ability to deform and pass through narrow capillaries. This leads to cell rupture and hemolysis. Leukocytes utilize surface carbohydrates to regulate their movement throughout the body, especially during inflammatory responses. For instance, the sialylated LewisX antigen located on leukocyte surfaces binds with selectins on endothelial cells during acute inflammation. This interaction facilitates leukocyte rolling and adhesion to blood vessel walls, allowing the cells to migrate to infection sites.
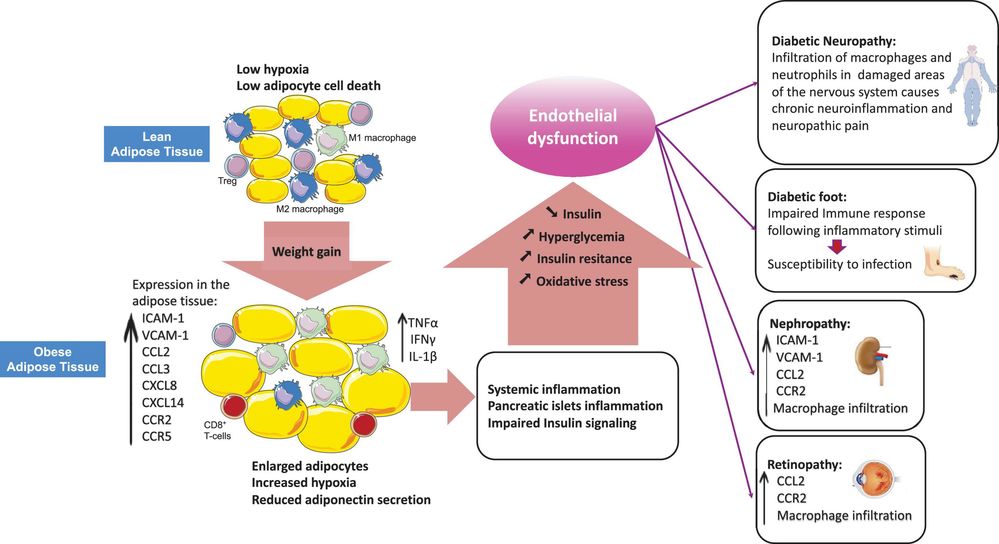 Fig.2 Obesity-Induced disruption of leukocyte trafficking in type 2 diabetes mellitus (T2DM).2,3
Fig.2 Obesity-Induced disruption of leukocyte trafficking in type 2 diabetes mellitus (T2DM).2,3
Our Services: Cell Function Testing
Creative Biolabs provides cell function testing services that analyze erythrocyte deformability together with leukocyte chemotaxis and adhesion capabilities. These tests provide valuable insights into disease diagnosis and therapy, helping to understand the physiological and pathological changes occurring in blood cells.
Carbohydrate Chains in Immune Recognition by Blood Cells
Immune recognition depends on carbohydrate chains because they help the immune system differentiate self-cells from non-self entities. Immune cells use multiple receptors to detect distinct carbohydrate patterns, which subsequently activate immune responses.
Pattern Recognition by Immune Cells
Macrophages possess receptors that allow them to identify carbohydrate patterns present on the surfaces of pathogens. The mannose receptor on macrophages binds to glycans that contain high concentrations of mannose sugar, commonly found on bacterial surfaces. This interaction leads to phagocytosis and triggers immune responses.
Self & Non-Self Discrimination
The immune system uses carbohydrate chains to differentiate between self and non-self entities. For instance, during transplant rejection, the immune system identifies foreign carbohydrate structures on donor blood cells, triggering an immune attack. Conversely, the immune system recognizes the glycans on self-blood cells as non-threatening and leaves them intact.
Our Services: Immune Cell Testing
Creative Biolabs offers specialized services to assess how immune cells recognize various carbohydrate structures during immune cell testing. Our services deliver essential data that supports the development of vaccines, immune therapies, and other immunological treatments.
Altered Carbohydrate Chains Affect Blood Cell Functionality
Disease mechanisms in conditions such as cancer and autoimmune disorders often involve changes in blood cell glycosylation patterns (carbohydrate chains in cancer). Altered glycosylation can have significant therapeutic implications. Modifications to carbohydrate chains frequently occur in various health conditions including cancer and autoimmune diseases. For instance, breast tumor cells often exhibit elevated levels of sialylation in their surface glycans, which support both cell proliferation and metastasis. The glycosylation changes that occur on leukocytes during rheumatoid arthritis lead to abnormal immune responses, resulting in attacks on healthy tissues. Altered glycosylation patterns in blood cells present a promising treatment strategy for a range of diseases. Antibodies designed to target tumor-linked glycans represent a novel approach in cancer treatment, aiming to disrupt the pathways that enable tumor growth and metastasis. Additionally, treatments for autoimmune diseases that adjust leukocyte glycosylation patterns hold promise for reducing improper immune system activity.
Research Progress in Carbohydrate Chains of Blood Cells
Ongoing research is uncovering new insights into the role of glycans in blood cells. The study of glycosylation patterns and their implications for disease and therapy is an area of active investigation. Creative Biolabs provides a wide range of high-quality blood group products, including but not limited to Lewisa, Lewisb, Lewisx, and Lewisy trisaccharides, tetrasaccharides, and pentasaccharides, as well as blood group A, blood group B, and blood group H trisaccharides, tetrasaccharides, and pentasaccharides.
Advances in Research on Blood Group Carbohydrate Chains
The study of human red blood cell blood group ABH determinant clusters continues to progress. It is known that these clusters are carried by four types of glycolipid carbohydrate chains. The development of these carbohydrate chains varies throughout an individual's life. For example, complex variants of the A or H determinant clusters are either absent or significantly reduced in fetal and neonatal red blood cells, whereas these structures are fully developed in adult red blood cells. In contrast, the simpler carbohydrate chain-linked A determinant clusters are fully developed before birth and show little change postnatally. Additionally, studies have shown that blood group carbohydrate chains gradually extend and branch during individual development, but this process is disrupted during tumorigenesis.
Advances in Universal Blood Preparation Research
Enzymes derived from the intestinal bacterium Ackermannia species show significant promise. Research has revealed that these enzymes can convert both known and unknown antigens on human red blood cells, effectively converting A and B type red blood cells to type O blood. This method is also effective on newly discovered expanded forms of A and B blood types and significantly reduces mismatched reactions in tests. This breakthrough provides a promising solution for increasing the supply of universally compatible blood.
Advances in Leukemia Cell Carbohydrate Chain Research
Significant progress has been made in the study of HL-60 cells. When HL-60 leukemia cells are induced to differentiate into granulocytes or monocytes, noticeable changes occur in the N-glycan structures on their surfaces, along with alterations in the activity of related N-acetylglucosamine transferases (GNT-III, GNT-VI) and α1,6 fucosyltransferases (α1, Fuct). Research has revealed the enzymatic mechanisms behind the reduction in antennae number and core fucose, as well as the increase or decrease in bisected GlcNAc. Furthermore, studies have explored the relationship between the differentiation direction and the expression and distribution changes of related compounds and protein kinase C subtypes, offering new avenues and directions for leukemia treatment and research.
References
-
Raposo, Cláudia D., André B. Canelas, and M. Teresa Barros. "Human lectins, their carbohydrate affinities and where to find them." Biomolecules 11.2 (2021): 188. https://doi.org/10.3390/biom11020188
-
Pezhman, Laleh, Abd Tahrani, and Myriam Chimen. "Dysregulation of leukocyte trafficking in type 2 diabetes: mechanisms and potential therapeutic avenues." Frontiers in cell and developmental biology 9 (2021): 624184. https://doi.org/10.3389/fcell.2021.624184
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Structural variations of carbohydrate building blocks in nature.1,3
Fig.1 Structural variations of carbohydrate building blocks in nature.1,3
 Fig.2 Obesity-Induced disruption of leukocyte trafficking in type 2 diabetes mellitus (T2DM).2,3
Fig.2 Obesity-Induced disruption of leukocyte trafficking in type 2 diabetes mellitus (T2DM).2,3

