Why Carbohydrate Chains Matter in Microbial Infections?
Carbohydrate chains (glycans) function as crucial molecular structures that determine microbial survival and pathogenicity while mediating interaction with host immune systems. Glycans that cover bacterial, viral, and fungal surfaces act as flexible platforms to help microbes both identify targets and avoid detection. Microbial glycosylation represents a post-translational modification mechanism in which enzymes link carbohydrate chains to proteins or lipids. The glycosylation process extends beyond structural functions to operate as a microbial survival mechanism. Bacterial cell walls contain carbohydrate chains, including lipopolysaccharides in Gram-negative bacteria and teichoic acids in Gram-positive bacteria which give mechanical stability and guard against external antimicrobial peptides. Pathogen glycoproteins including the SARS-CoV-2 spike protein undergo extensive glycosylation to maintain structural stability while enabling cellular entry into host cells. Creative Biolabs offers glycan profiling services that allow scientists to decipher complex glycan structures to discover pathogen-specific glycosylation patterns, which are important for developing vaccines and therapies.
The Dual Role of Carbohydrate Chains: Recognition vs. Manipulation
Immune System Recognition
The carbohydrate chains in immune system recognition are central to host defense. Immune cells employ lectins, such as C-type lectin receptors (CLRs), to detect pathogen-associated glycans. For instance, mannose-binding lectin (MBL) binds to high-mannose glycans on bacterial surfaces, triggering the complement system to lyse pathogens. This pathogen recognition by carbohydrate chains is a first-line defense mechanism. However, pathogens counter this through carbohydrate chains in immune evasion. Neisseria meningitidis, for example, caps its LPS with sialic acid residues that mimic host glycans, effectively "hiding" from complement-mediated destruction.
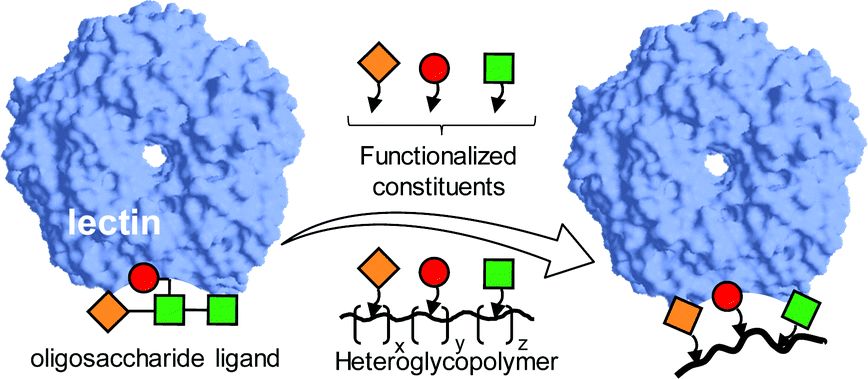 Fig. 1 Synthetic heteroglycopolymers for lectin recognition and binding.1
Fig. 1 Synthetic heteroglycopolymers for lectin recognition and binding.1
Microbial Immune Evasion
Viruses like HIV-1 exploit carbohydrate chains and immune escape by densely glycosylating their envelope proteins. The "glycan shield" on HIV's gp120 protein sterically hinders antibody access to conserved epitopes, a strategy also observed in influenza hemagglutinin. Creative Biolabs' glycoengineering platform supports the development of antibodies that penetrate these shields by targeting rare, unshielded regions.
How Pathogens Use Carbohydrate Chains to Infect Hosts
Carbohydrate Chains in Bacterial Cell Walls and Adhesion
The carbohydrate side chains in bacteria are not passive components. In E. coli, fimbriae tipped with lectin-like adhesins bind to host glycans (e.g., galactose residues on urothelial cells), enabling urinary tract colonization. Similarly, Helicobacter pylori uses blood group antigen-binding adhesin (BabA) to attach to Lewis b glycans in the gastric mucosa. Creative Biolabs offers polysaccharides with antibacteria activity identification service to help identify polysaccharides that target bacterial adhesion, offering a strategic approach for developing anti-infective therapies.
Viral Infections and Glycan-Mediated Host Entry
Carbohydrate chains in viral infections are gatekeepers for host cell entry. The SARS-CoV-2 spike protein's N-linked glycans stabilize its receptor-binding domain (RBD), while O-linked glycans on the S2 subunit modulate membrane fusion. HIV's gp120 binds to host CD4 via high-mannose glycans, a process modeled using pseudovirus entry assays.
Microbial Glycosylation: Camouflage and Deception
Pathogens like Campylobacter jejuni mimic host gangliosides through microbial glycosylation in infections, triggering autoimmune responses (e.g., Guillain-Barré syndrome). Candida albicans masks β-glucans with mannoproteins to evade Dectin-1 recognition. At Creative Biolabs, we specialize in comprehensive glycosylation analysis services for virus glycoproteins, offering in-depth analysis of glycan structures on viral glycoproteins. Our services support viral research, including for specific pathogens like COVID-19, SARS-CoV, MERS-CoV, and HIV. Whether you're studying hepatitis C, Ebola, or Dengue, our detailed glycosylation analysis helps guide the development of therapeutic and vaccine strategies. Explore our services for Influenza, Chikungunya, and more.
Targeting Carbohydrate Chains for Disease Resistance
Pathogen carbohydrate chain targeting represents a promising method for improving disease resistance. Researchers are investigating glycan-based therapeutics and vaccines alongside diagnostic tools as potential solutions for fighting microbial infections and other associated diseases. The extensive variety and precise nature of glycan structures enable precise medical interventions, which become highly beneficial for addressing antimicrobial resistance (AMR) and avoiding pathogen immune evasion tactics.
Glycan Profiling: Revolutionizing Infection Diagnostics
Glycan profiling requires thorough analysis of microbial surface glycan structures which function as indicators for infections and resistance to therapies. Multiple research studies have validated glycan-based diagnostic methods for detecting pathogens early and identifying patterns of antibiotic resistance. The surface glycans of Pseudomonas aeruginosa play a dual role in both causing disease and providing resistance against the antibiotic colistin. Research indicates that alterations in LPS structure serve as indicators of colistin resistance. These developments demonstrate how glycan signatures function as essential tools for infection diagnosis and treatment planning in cases of multi-drug-resistant bacterial strains. Glycomic profiling serves as a tool to detect pathogen-specific glycosylation patterns which enable pathogens to avoid detection by host immune systems. Research from 2021 identified particular glycan formations on Streptococcus pneumoniae surfaces that relate to both bacterial virulence and its ability to avoid host immune detection. The following table lists pathogen-specific glycan markers which serve important functions in diagnostics and infection control.
|
Pathogen
|
Glycan Marker
|
Role in Pathogenesis
|
|
Pseudomonas aeruginosa
|
LPS (Lipopolysaccharide)
|
Antibiotic resistance, biofilm formation
|
|
Streptococcus pneumoniae
|
Capsular polysaccharides
|
Virulence, immune evasion
|
|
Escherichia coli
|
Fimbrial adhesins (type 1 fimbriae)
|
Urinary tract adhesion
|
|
Helicobacter pylori
|
Lewis b antigens
|
Gastric mucosal adhesion
|
These advancements in glycan profiling have led to the development of platforms that utilize mass spectrometry, lectin arrays, and other glycomics tools to rapidly identify pathogens and their resistance mechanisms. Creative Biolabs' glycoprotein structure analysis services provide cutting-edge technology to facilitate this process and enhance clinical diagnostics.
Glycoconjugate Vaccines and Anti-Adhesion Therapies
Glycoconjugate vaccines, which link carbohydrate antigens from pathogens to protein carriers, have proven effective in boosting immune responses against infections. These vaccines not only induce the production of antibodies against the specific glycan antigens but also facilitate the activation of T-helper cells, resulting in broader protection against microbial pathogens. For example, the pneumococcal conjugate vaccine (PCV) is designed to prevent infection by Streptococcus pneumoniae, which contains a polysaccharide capsule that is crucial for its virulence. By conjugating this polysaccharide to a protein carrier such as diphtheria toxoid, the immune system is primed to recognize the pathogen more efficiently. Similarly, meningococcal vaccines utilize conjugated polysaccharides to protect against Neisseria meningitidis. There are some comparisons of glycoconjugate vaccines in use and their target pathogens.
|
Vaccine Type
|
Target Pathogen
|
Glycan Target
|
Immunization Strategy
|
|
Pneumococcal Conjugate
|
Streptococcus pneumoniae
|
Capsular polysaccharides
|
Conjugated to diphtheria toxoid or similar proteins
|
|
Meningococcal Conjugate
|
Neisseria meningitidis
|
Meningococcal polysaccharides
|
Conjugated to tetanus toxoid
|
|
Haemophilus B Conjugate
|
Haemophilus influenzae
|
Polyribosylribitol phosphate (PRP)
|
Conjugated to tetanus toxoid or similar proteins
|
The strategy behind these vaccines involves carbohydrate-based immunity, where the immune system recognizes the unique structures of microbial glycans, triggering a robust and long-lasting immune response. This approach can also be applied to new emerging pathogens, including antimicrobial-resistant strains. Anti-adhesion therapies further complement this strategy by preventing pathogens from adhering to host cells. Adhesion to epithelial cells is the first step in many infections, and by disrupting this process, the spread of infection can be minimized. D-mannose, for example, has been shown to inhibit the adhesion of E. coli to urinary tract cells, preventing urinary tract infections (U.T.I.s). Similarly, other anti-adhesion agents are being explored to block pathogen binding to mucosal surfaces, effectively reducing infection rates and enhancing patient outcomes.
Glycoengineering: Disrupting Pathogen Glycosylation
Glycoengineering involves manipulating the glycosylation pathways in both pathogens and host cells to disrupt pathogenic processes and immune evasion strategies. One of the most exciting advancements in this field is the development of synthetic glycans that can be used to block pathogen-host interactions. For example, glycan-based inhibitors that prevent HIV from binding to host CD4 receptors are under investigation. These inhibitors can be designed to mimic natural glycan structures on the surface of the host cell, preventing viral attachment.
Moreover, Gene-editing technology is being utilized to modify the glycosylation machinery in pathogens, reducing their virulence (bacteria glycoengineering services). By knocking out specific glycosyltransferase genes, researchers can attenuate the pathogen's ability to synthesize crucial glycan structures that are necessary for immune evasion. For instance, a study in 2019 has demonstrated that the deletion of specific glycosylation enzymes in Pseudomonas aeruginosa impaired its biofilm formation and reduced its pathogenicity in animal models. Creative Biolabs' high-throughput glycan screening service facilitates high-throughput screening of glycan-binding inhibitors and help identify novel compounds that target key glycan structures involved in infection.
Reference
-
González-Cuesta, Manuel, Carmen Ortiz Mellet, and José M. García Fernández. "Carbohydrate supramolecular chemistry: Beyond the multivalent effect." Chemical Communications 56.39 (2020): 5207-5222. Distributed under Open Access license CC BY 3.0, without modification. https://doi.org/10.1039/D0CC01135E
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig. 1 Synthetic heteroglycopolymers for lectin recognition and binding.1
Fig. 1 Synthetic heteroglycopolymers for lectin recognition and binding.1

