How Carbohydrate Chains Shape Hormone Communication
Carbohydrate chains in hormone regulation act as dynamic "sugar antennas" that fine-tune cellular communication. These glycans, covalently attached to hormones or their receptors, modulate ligand-receptor interactions, signal transduction efficiency, and downstream metabolic responses. For instance, insulin receptors rely on glycosylation of hormone receptors to maintain structural integrity and ligand-binding specificity. The insulin receptor's extracellular domain contains N-linked glycans that stabilize its conformation, enabling precise "listening" to circulating insulin levels. Studies show that deglycosylation of follicle-stimulating hormone (FSH) reduces its binding affinity by 50%, while sialylation of luteinizing hormone (LH) prolongs its serum half-life by shielding it from hepatic clearance. This highlights how glycans and hormone signaling synergize to balance endocrine activity.
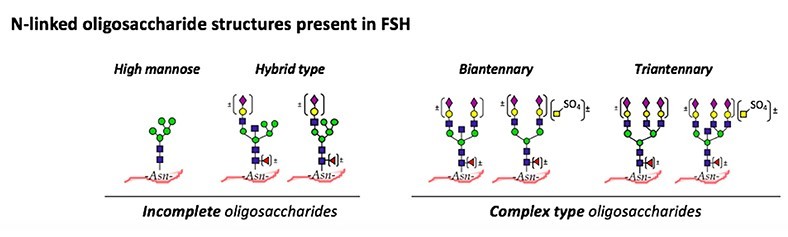 Fig.1 N-linked oligosaccharide on human FSH.1,4
Fig.1 N-linked oligosaccharide on human FSH.1,4
In the endocrine system glycosylation, carbohydrate chains function as molecular ID badges. Thyroid-stimulating hormone (TSH), for example, carries sialylated N-glycans that determine its metabolic stability and target specificity. Aberrant glycosylation of TSH is linked to hypothyroidism, where undersialylated isoforms exhibit reduced bioactivity and accelerated clearance. Similarly, thyroglobulin (Tg), the precursor of thyroid hormones T3 and T4, requires precise N-glycosylation at Asn57 and Asn91 for proper iodination and hormone release. These findings underscore the role of glycans in hormone stability and signaling. For advanced glycosylation profiling, Creative Biolabs provides services like high-throughput glycan screening and glycosylation site mapping to evaluate glycosylation alterations and their impact on hormonal function, enabling the identification of glycosylation patterns crucial for insulin signaling.
Carbohydrate Chains in Insulin Signaling: The Glucose Gatekeeper
Carbohydrate chains in the regulation of insulin signaling act as gatekeepers of glucose homeostasis. The insulin receptor's α-subunit contains critical N-glycosylation sites (e.g., Asn25) that facilitate dimerization and autophosphorylation. Hyperglycemia-induced impact of glycosylation on insulin resistance occurs when excessive glucose flux through the hexosamine biosynthetic pathway increases O-GlcNAcylation of IRS-1, disrupting insulin receptor substrate phosphorylation. Key mechanisms include:
-
Glycosylation of hormone receptors: High glucose levels promote aberrant branching of N-glycans on the insulin receptor, reducing its affinity for insulin by 30–40%.
-
Oxidative stress: Advanced glycation end products (AGEs) form crosslinks with receptor glycans, impairing conformational flexibility.
With glycosylation profiling services to identify such pathological modifications in insulin signaling pathways, Creative Biolabs also provides a comprehensive suite of monosaccharides analysis, polysaccharides analysis and oligosaccharides analysis to evaluate modifications in glycosylation that may influence thyroid hormone activity.
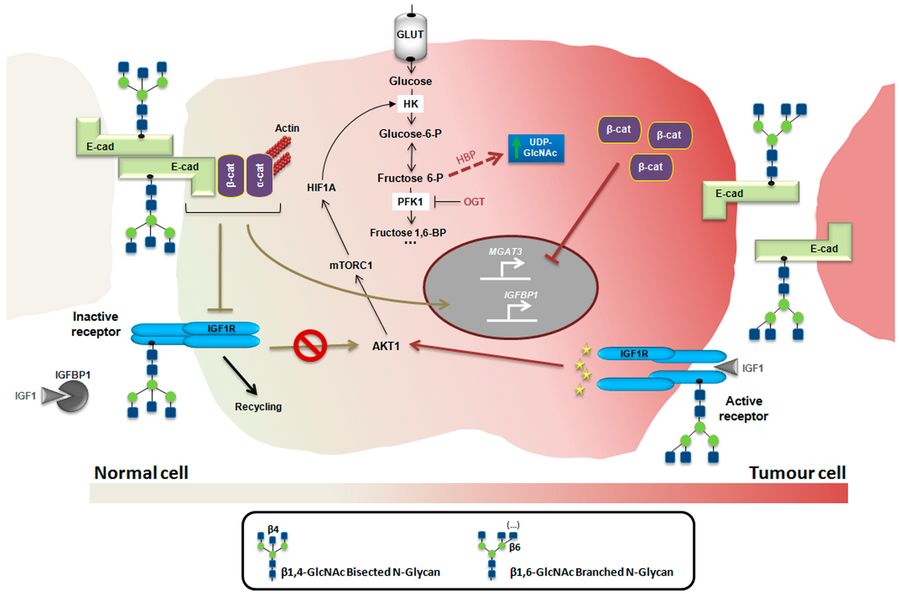 Fig.2 Glycans in insulin/IGF system.2,4
Fig.2 Glycans in insulin/IGF system.2,4
Glycans and Glycosylation in Thyroid Hormone
Thyroid hormones, packaged as energy metabolism regulators, depend on glycosylation of hormone receptors for targeted delivery. The sodium/iodide symporter (NIS) on thyrocytes requires N-glycans for proper membrane trafficking, ensuring iodide uptake—a rate-limiting step in T3/T4 synthesis. Hypothyroidism cases often correlate with undersialylated TSH receptors, which exhibit 60% reduced cAMP activation compared to fully glycosylated isoforms.
Changes to thyroid hormones, called glycosylation, are important for how the thyroid works, and for thyroid diseases to develop. These changes affect the structure and function of thyroid hormones. They may also play a role in the development of diseases like thyroid cancer. Thyroglobulin (Tg) is the main precursor of thyroid hormones, and its N-glycosylation takes place at multiple sites, including Asn59 and Asn57. N-glycosylation affects the structure and stability of Tg. It also affects its movement within thyroid cells and secretion. There are other changes to thyroid hormones that also have important effects on how they work and how they are processed. These changes include sialylation, sulfation, 1,6-branching structure, poly-N-acetylglucosamine chain length, and O-glycosylation. For example, changes in the structure of thyroid hormones can affect how well they work and how quickly they are broken down. In addition, changes in thyroid hormones have been linked to the development of thyroid cancer. Studies have shown significant structural changes in thyroid hormones in patients with thyroid cancer, including increased salivary acidification and sulfation. These changes may be linked to the growth and spread of thyroid cancer cells.
Autoimmune thyroid disorders like Hashimoto's thyroiditis involve glycan modifications in hormone resistance mechanisms. Anti-thyroglobulin IgG antibodies in Hashimoto's patients show hypo-fucosylation, which enhances immune complex formation and thyroid destruction. Equipped with advanced platforms for glycan analysis, Creative Biolabs' glycoprotein analysis services enable precise analysis of such glyco-immune interactions.
Precision Glycoengineering and Biomarkers Development in Hormone Therapy
Recombinant hormones like erythropoietin (EPO) benefit from glycoengineering to optimize half-life. Sialylation of EPO's N-glycans increases its circulatory persistence from 4 hours to 24 hours, reducing dosing frequency in anemia treatment. Similarly, glucagon-like peptide-1 (GLP-1) analogs with engineered glycans show enhanced stability for type 2 diabetes management. To support these developments, Creative Biolabs provides glycoengineering services and glyco-engineered mammalian cell expression system, glyco-engineered pichia pastoris expression system and glyco-engineered plant-based expression system to develop optimized production systems for glycoprotein-based therapies. Additionally, glycan remodeling services allow researchers to precisely adjust glycosylation patterns, enhancing therapeutic potential in metabolic diseases.
Carbohydrate chains in hormone regulation are indispensable for endocrine precision, influencing hormone stability, receptor activation, and metabolic outcomes. Dysregulated glycosylation of hormone receptors underpins pathologies like insulin resistance and thyroid dysfunction, necessitating advanced glycoanalytical tools. To further explore glycosylation changes in thyroid-related diseases like thyroid cancer, cancer glycomics analysis services from Creative Biolabs provide in-depth profiling of glycan alterations in thyroid tissues. For example, core fucosylation of TSH glycans serves as a biomarker for early-stage hypothyroidism. Besides, glycoprotein detection techniques can be applied to identify glycoproteins involved in thyroid dysfunction.
Published Data
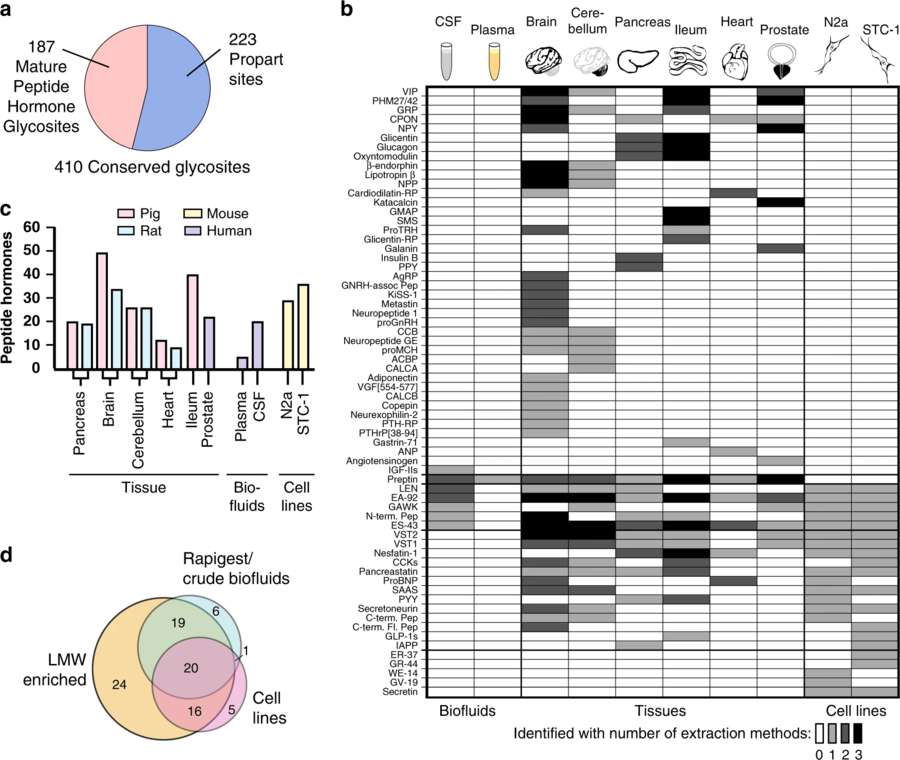 Fig.3 Mapping O-Glycosylation on Peptide Hormones Using LC–MS/MS.3,4
Fig.3 Mapping O-Glycosylation on Peptide Hormones Using LC–MS/MS.3,4
This study explores O-glycosylation modifications on peptide hormones using various techniques and experimental methods, including mass spectrometry (LC–MS/MS), low molecular weight enrichment (LMWE), lectin weak affinity chromatography (LWAC), and bioinformatics analysis. The researchers found that O-glycosylation at receptor-binding regions can regulate receptor activation characteristics and significantly extend the half-life of peptide hormones. Additionally, O-glycosylation may modulate their biological activity by affecting the structural stability of the peptide hormones. The above-mentioned figure presents the results of analyzing the O-glycosylation sites of peptide hormones using LC–MS/MS technology. Approximately 97,000 peptide spectra with glycosylation information were matched from various biological samples, with around 410 conserved O-glycosylation sites identified. These sites are distributed across 91 mature peptides from 279 known peptide hormones. The figure also illustrates the distribution of glycosylated peptide hormones identified in different biological samples and highlights the importance of LMWE technology in improving identification efficiency. The application of LC–MS/MS technology is crucial for revealing the O-glycosylation profiles of peptide hormones, as it can sensitively detect low-abundance glycosylation modifications and provide a foundation for subsequent functional studies.
References
-
Campo, Stella, et al. "Hormonal regulation of follicle-stimulating hormone glycosylation in males." Frontiers in endocrinology 10 (2019): 17. The image retrieved from Figure 1 " Hormonal regulation of follicle-stimulating hormone glycosylation in males." Campo, Stella, 2019. The image has been cropped, retaining only part B. https://doi.org/10.3389/fendo.2019.00017
-
de-Freitas-Junior, Julio Cesar M., et al. "Glycans as regulatory elements of the insulin/IGF system: impact in cancer progression." International Journal of Molecular Sciences 18.9 (2017): 1921. https://doi.org/10.3390/ijms18091921
-
Madsen, Thomas D., et al. "An atlas of O-linked glycosylation on peptide hormones reveals diverse biological roles." Nature Communications 11.1 (2020): 4033. https://doi.org/10.1038/s41467-020-17473-1
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 N-linked oligosaccharide on human FSH.1,4
Fig.1 N-linked oligosaccharide on human FSH.1,4
 Fig.2 Glycans in insulin/IGF system.2,4
Fig.2 Glycans in insulin/IGF system.2,4
 Fig.3 Mapping O-Glycosylation on Peptide Hormones Using LC–MS/MS.3,4
Fig.3 Mapping O-Glycosylation on Peptide Hormones Using LC–MS/MS.3,4

