Why Carbohydrate Chains Matter in Cellular Stress
Carbohydrates, often in the form of carbohydrate chains, play a pivotal role in cellular stress response mechanisms. Glycosylation, the covalent attachment of carbohydrate chains to proteins and lipids, is a critical post-translational modification that can influence protein function, stability, and interactions. In cellular stress, such as oxidative stress, heat shock, or endoplasmic reticulum (ER) stress, glycosylation can modulate the activity of numerous cellular pathways. Carbohydrate chains in stress response pathways directly impact several molecular mechanisms, such as protein folding, the unfolded protein response (UPR), and regulation of cell signaling. Glycosylation is crucial for the activation or suppression of proteins involved in endoplasmic reticulum stress, oxidative stress, DNA damage repair, and apoptosis. By modifying protein structure, glycosylation enables proteins to interact with cellular components more effectively, which is essential for maintaining cellular homeostasis under stress (as shown in the following table). These processes are critical in determining whether a cell survives or undergoes programmed cell death in response to stressors.
|
Stress Type
|
Role of Glycosylation
|
Example Proteins/Processes
|
|
Oxidative Stress
|
Protects against reactive oxygen species (ROS), stabilizes antioxidants
|
Antioxidant enzymes, Nrf2 signaling
|
|
Endoplasmic Reticulum Stress
|
Modulates UPR pathways, controls protein folding and misfolding responses
|
GRP78, IRE1, and ATF6
|
|
DNA Damage
|
Facilitates DNA repair processes and genomic stability
|
PARP1, DNA repair enzymes
|
|
Apoptosis
|
Regulates cell survival or death pathways via death receptors and caspases
|
Fas, TRAIL, and caspase-3 activation
|
Creative Biolabs offers custom carbohydrate analysis services and identification and characterization of protein glycosylation services to identify and characterize carbohydrate chains in stress models, enabling the development of therapies that manipulate glycosylation for enhanced stress resilience or to promote cell death in diseases like cancer. We also provide glycoengineering services to optimize glycan structures in therapeutic glycoproteins, improving stability and functionality under stress conditions where protein misfolding occurs.
Carbohydrate Chains in Specific Stress Conditions
Carbohydrate Chains in Heat Shock Proteins (HSPs)
Heat shock proteins (HSP70, HSP90, etc.) are molecular chaperones that regulate protein folding, prevent aggregation, and rescue misfolded proteins. Carbohydrate chains critically modulate HSP functions through three mechanisms:
-
Structural stability: Glycosylation stabilizes HSP conformation during thermal/oxidative stress.
-
Client interaction: Carbohydrate moieties expand HSP binding capacity for diverse misfolded proteins (e.g., glycosylated HSP90 recruits co-chaperones 20% faster).
-
Stress adaptation: In hypoxia, HSP70 glycoforms show 1.5-fold higher ATPase activity to sustain proteostasis.
Oxidative Stress and DNA Damage Repair
Reactive oxygen species (ROS) damage proteins, lipids, and DNA. Carbohydrate chains mitigate oxidative stress through dual pathways:
|
Target
|
Glycosylation Effect
|
Outcome
|
|
Nrf2 pathway
|
Stabilizes Keap1-Nrf2 complex
|
Upregulates antioxidant genes (e.g., SOD, CAT)
|
|
PARP1
|
Enhances DNA binding affinity by 40% via O-GlcNAcylation
|
Accelerates DNA strand break repair
|
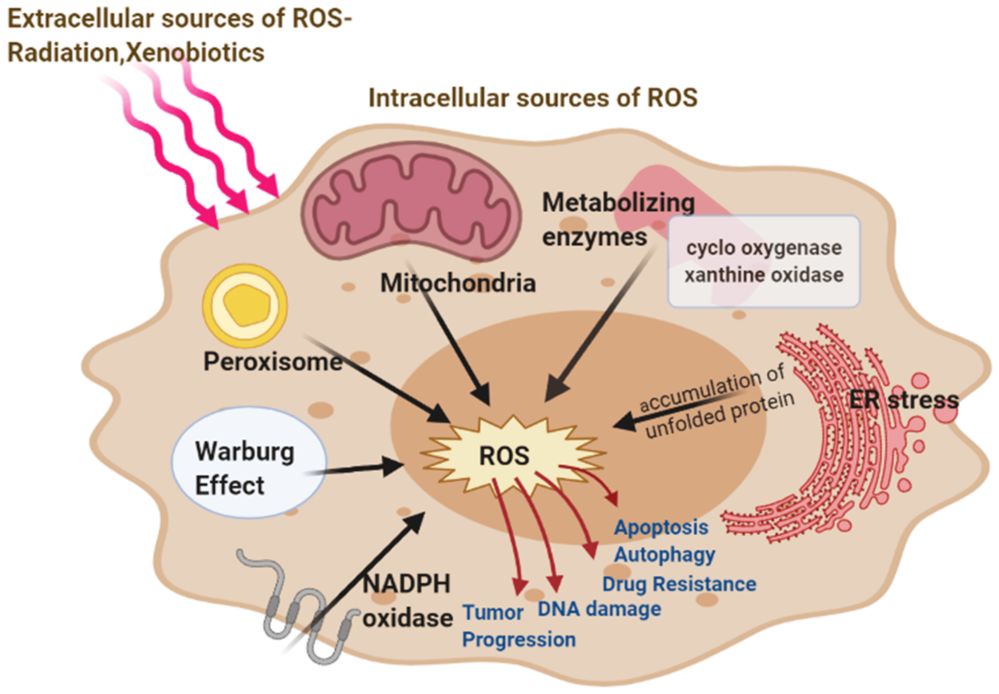 Fig.1 Major intracellular and extracellular sources of ROS.1,3
Fig.1 Major intracellular and extracellular sources of ROS.1,3
Endoplasmic Reticulum Stress: Glycan-Regulated Fate Switching
ER stress triggers the unfolded protein response (UPR). Key glycosylation nodes include:
|
Pro-survival phase
|
-
GRP78 glycosylation maintains chaperone activity under nutrient deprivation
-
IRE1α glycoforms enhance XBP1 splicing efficiency
|
|
Pro-apoptotic shift
|
-
Hyperglycosylated TRAIL receptors increase caspase-8 activation by 3-fold
-
Deglycosylation of ATF6 promotes CHOP-mediated apoptosis
|
Glycoengineering Strategies for Stress Adaptation
Therapeutic Glycoprotein Optimization
-
Charge modulation: Sialylation increases serum half-life (e.g., α1-acid glycoprotein stability +60% at 42°C)
-
Targeted delivery: Mannose-6-phosphate glycans direct lysosomal enzymes via CI-MPR receptors
At Creative Biolabs, we offer specialized therapeutic protein glycoengineering services for therapeutic proteins, including Bio-better glucocerebrosidase glycoengineering service and Bio-better glucarpidase glycoengineering service, tailored to enhance efficacy and stability. We also provide therapeutic antibody glycoengineering services, focusing on optimizing antibody glycosylation for improved efficacy. The custom glycosyl linker synthesis service provides bespoke linker solutions for glycoproteins, while our glycan remodeling service enables modifications to glycan structures for enhanced biological activity.
Genetic Control of Glycosylation
|
System
|
Technique
|
Application Example
|
|
Plant/microalgae
|
Gene-edited GnTIII overexpression
|
Salt stress-resistant lectin production
|
|
Mammalian cells
|
MGAT1-KO CHO cells
|
Homogeneous antibody glycosylation (PD-L1 inhibitor)
|
Here are some of our related services that can help you with genetic glycoengineering and cell line glycoengineering:
Published Data
Inhibition of Glycosylation by ManN and Stress Pathway Activation
The following figure provides critical insights into how glycosylation, specifically through the action of ManN (D-mannosamine), modifies proteins to regulate stress pathways in endothelial cells. Glycosylation is a post-translational modification that involves the addition of carbohydrate groups to proteins, playing a crucial role in protein folding, stability, and function. In this study, ManN is shown to inhibit glycosylation, leading to significant changes in the molecular mass of key proteins such as VEGFR2, Neuropilin-1, CD31, and c-Met. These changes are indicative of altered glycan structures, which can disrupt protein folding and stability, thereby activating stress pathways. The data demonstrate that ManN specifically reduces the molecular mass of VEGFR2 in a dose-dependent manner, with new lower molecular weight bands appearing. This effect is unique to ManN among the tested hexosamines and can be reversed by mannose, suggesting a competition for cellular uptake or incorporation into glycan structures. The reversibility of these changes further underscores the dynamic nature of glycosylation in regulating protein function. Additionally, the activation of stress pathways, such as the unfolded protein response (UPR), is likely a cellular response to the misfolded proteins resulting from glycosylation inhibition. This study highlights the intricate interplay between glycosylation and stress pathways, revealing a novel mechanism by which ManN can modulate endothelial cell behavior and potentially promote angiogenesis through stress pathway activation.
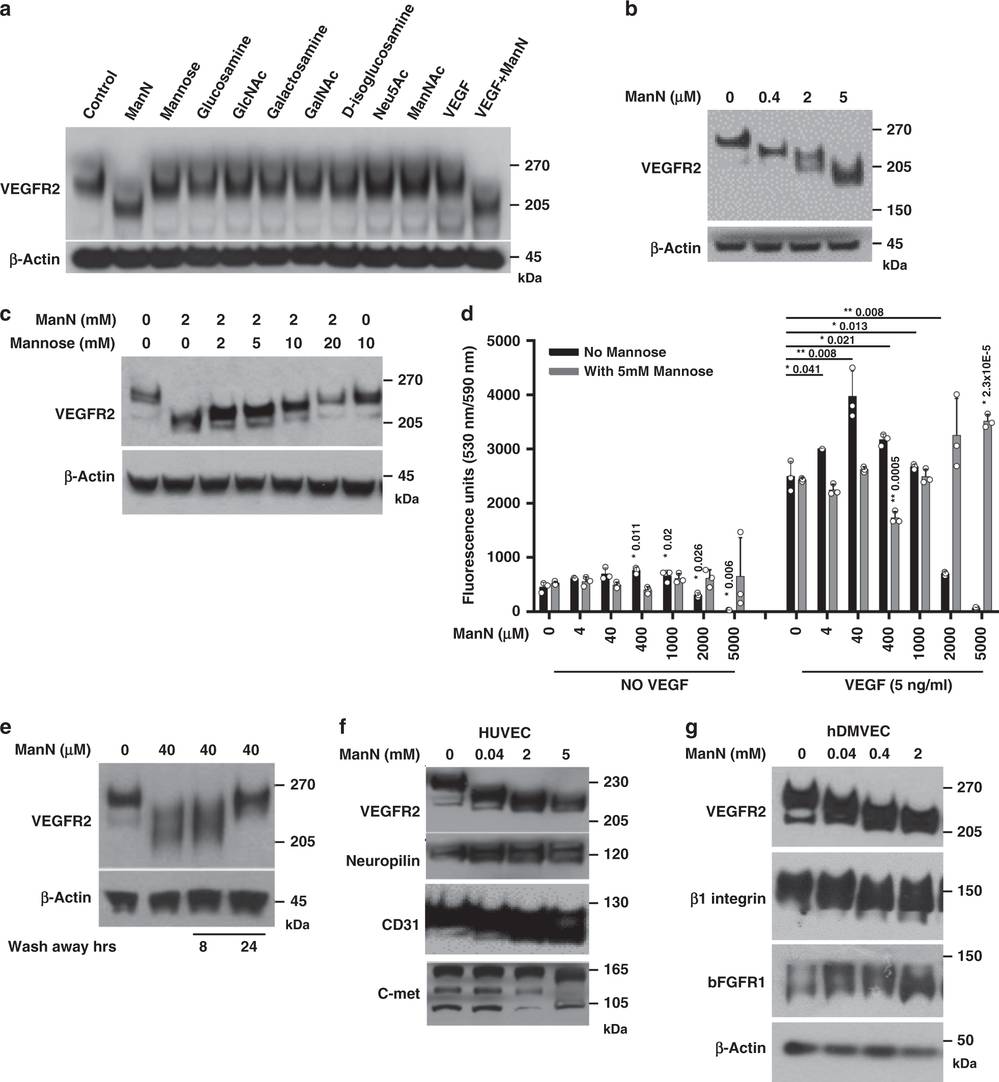 Fig.2 ManN-induced glycosylation changes and protein modifications.2,3
Fig.2 ManN-induced glycosylation changes and protein modifications.2,3
References
-
Arfin, Saniya, et al. "Oxidative stress in cancer cell metabolism." Antioxidants 10.5 (2021): 642. https://doi.org/10.3390/antiox10050642
-
Zhong, Cuiling, et al. "Inhibition of protein glycosylation is a novel pro-angiogenic strategy that acts via activation of stress pathways." Nature Communications 11.1 (2020): 6330. https://doi.org/10.1038/s41467-020-20108-0
-
Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Major intracellular and extracellular sources of ROS.1,3
Fig.1 Major intracellular and extracellular sources of ROS.1,3
 Fig.2 ManN-induced glycosylation changes and protein modifications.2,3
Fig.2 ManN-induced glycosylation changes and protein modifications.2,3

