Carbohydrate Chains in Stem Cell Identity and Differentiation
Stem cells are the body's master builders, capable of transforming into any cell type. But how do these cells "know" their identity or decide their fate? The answer lies in carbohydrate chains—sugar molecules that act as cellular barcodes and molecular switches. These glycostructures critically modulate stem cell behavior by directing identity maintenance and differentiation trajectories. Notably, glycosylation events and carbohydrate chain modifications exert profound influences on pluripotency regulation, lineage commitment, and therapeutic applications in regenerative medicine.
Glycosylation as a Cellular Barcode
Every stem cell subtype carries unique carbohydrate chains on its surface, much like a fingerprint. Specific carbohydrate configurations, including hallmark pluripotency markers SSEA-3/4 and Tra-1-60/81, enable precise identification of pluripotent stem cells (PSCs) encompassing both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). These glycan signatures evolve dynamically during differentiation processes.
Cutting-edge mass spectrometric analyses have revealed differential sialylation patterns between ESC and iPSC populations. ESCs predominantly feature α-2,3-linked sialic acids, whereas iPSCs exhibit elevated α-2,6-linked variants—a distinction with critical implications for therapeutic quality control. Sialic acid modifications, prominent in glycoproteins and glycolipids, modulate essential cellular functions including signal transduction, intercellular adhesion, and immune interaction, thereby maintaining pluripotent states while priming differentiation readiness. At Creative Biolabs, our integrated glycomics platform synergizes advanced mass spectrometry with artificial intelligence to resolve stem cell glycosylation landscapes, enabling precise therapeutic characterization.
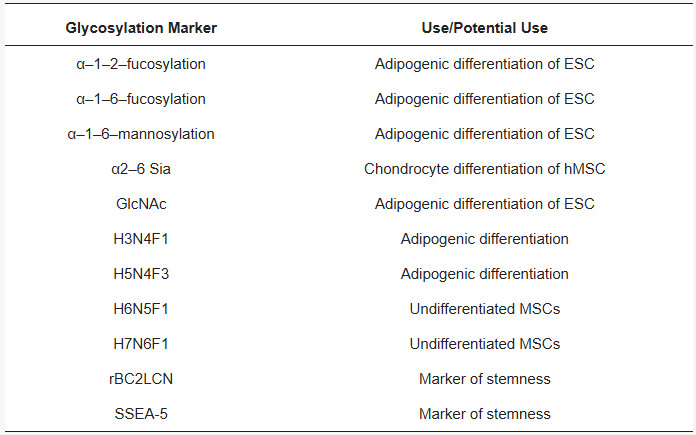 Fig.1 Examples of glycosylation use/potential use as markers in relation to stem cells and their differentiation.1
Fig.1 Examples of glycosylation use/potential use as markers in relation to stem cells and their differentiation.1
Mechanisms of Carbohydrate-Mediated Regulation of Differentiation
Carbohydrate chains don't just label cells—they actively control behavior:
-
Sialyl-LewisX (SLeX) modifications on hematopoietic stem cells (HSCs) regulate their differentiation via the Wnt/β-catenin pathway. Blocking SLeX synthesis forces HSCs into premature differentiation, compromising blood cell production.
-
GlcNAcylation, a dynamic sugar modification of proteins, stabilizes transcription factors like Oct4 and Nanog to maintain pluripotency. Inhibiting O-GlcNAc transferase (OGT) triggers spontaneous differentiation in ESCs within 48 hours.
Carbohydrate-Driven Strategies for Stem Cell Therapy and Regeneration
Glycan-mediated approaches are revolutionizing regenerative medicine through enhanced tissue repair and immunomodulatory capabilities, offering improved clinical outcomes in stem cell-based interventions.
Glycans in Tissue Engineering
Damaged tissues require scaffolds that mimic natural environments. Chitosan-hyaluronic acid hydrogels, enriched with chondroitin sulfate, replicate cartilage's extracellular matrix. When seeded with mesenchymal stem cells (MSCs), these scaffolds boost proliferation by 300% compared to traditional collagen matrices. By providing an optimized microenvironment that supports cell growth and differentiation, these scaffolds promote tissue regeneration, particularly in cartilage repair. Creative Biolabs offers Custom Chondroitin Sulfate Synthesis Service for your glycan-related research.
Carbohydrates in Stem Cell Immunomodulation
Beyond structural support, carbohydrates regulate immune compatibility in transplantation contexts. PD-L1 glycosylation exemplifies this mechanism, where α-2,6 sialic acid modifications attenuate natural killer (NK) cell cytotoxicity, enhancing transplanted stem cell survival. Such glycoimmune modifications present novel strategies to improve transplant success rates.
Carbohydrate-Driven Strategies for Stem Cell Homing and Engraftment
Carbohydrate-mediated homing mechanisms show particular promise in hematopoietic stem cell (HSC) therapies. Selectin-Sialyl-LewisX (SLeX) interactions significantly improve HSC recruitment to bone marrow niches. Recent breakthroughs demonstrate that FUT8 enzyme overexpression—enhancing glycoprotein fucosylation—boosts hepatocyte engraftment efficiency by 45% in liver regeneration models, underscoring the therapeutic potential of glycoengineered stem cells.
Challenges and Innovations in Carbohydrate-Based Stem Cell Engineering
Despite remarkable progress, glycoengineering faces critical hurdles requiring resolution. Structural heterogeneity in stem cell glycoprofiles introduces significant batch-to-batch variability, with glycan pattern coefficients of variation (CV) reaching 15%—a major obstacle for clinical standardization. Current research focuses on developing glycan profiling standardization protocols and machine learning-assisted quality control systems to ensure therapeutic consistency. This comprehensive integration of glycomics with stem cell biology promises to unlock new dimensions in precision regenerative medicine, bridging the gap between experimental models and clinical translation.
Glycan Homogenization with Targeted Gene Editing
Recent advances in genetic glycoengineering, particularly the use of targeted nuclease technologies, offer solutions to these challenges. By knocking out specific glycosyltransferase genes, such as FUT8, researchers can standardize glycosylation profiles, making them more uniform across different batches of stem cells. This process of glycan homogenization is expected to improve the consistency and predictability of stem cell therapies, bringing us closer to reliable clinical applications.
Cutting-Edge Technologies in Carbohydrate-Based Stem Cell Engineering
The future of carbohydrate-based stem cell engineering lies in the development of advanced technologies that can precisely control glycosylation and its effects on stem cell behavior.
-
3D-Bioprinted Glycan Gradients: Spatial control of sialic acid or heparan sulfate densities in scaffolds directs neural stem cells to differentiate into neurons (central regions) or glial cells (periphery), mimicking brain development.
-
Click Chemistry for Live Tracking: Metabolic labeling with azido-sugars allows real-time imaging of glycan migration in living organoids. Our platform achieves high resolution, revealing how glycans guide intestinal stem cell migration during gut repair.
Carbohydrates, along with their unique glycosylation patterns, are like the invisible conductors in the symphony of stem cell biology. They play an absolutely essential role in regulating stem cell identity, guiding their differentiation, and unlocking their remarkable therapeutic potential. Whether it's clearly defining the state of pluripotency, deftly controlling the destiny of stem cells, or significantly enhancing tissue regeneration, strategies centered around carbohydrates are right at the vanguard of stem cell engineering. However, we can't overlook the hurdles we face. Glycan heterogeneity, for instance, is like a complex maze that often confounds our efforts. But fear not! Innovative gene-editing techiques, the revolutionary 3D bioprinting, and the precise click chemistry are like powerful torches, illuminating the path towards more accurate and effective carbohydrate-based therapies.
Creative Biolabs stands at the very forefront of these exciting advancements. We're not just observers; we're active participants. We offer specialized services in stem cell research, including in-depth glycosylation site mapping service and advanced custom glycan synthesis services.Our aim is crystal-clear: to wholeheartedly support the development of next-generation stem cell therapies, bringing hope to patients who are waiting for medical breakthroughs.
Reference
-
Alghazali, Raghad, Ahmed Nugud, and Ahmed El-Serafi. "Glycan Modifications as Regulators of Stem Cell Fate." Biology 13.2 (2024): 76. Distributed under Open Access license CC BY 4.0, without modification.
Related Services
Resources
For Research Use Only.
 Contact Us
Follow us on
Contact Us
Follow us on

 Fig.1 Examples of glycosylation use/potential use as markers in relation to stem cells and their differentiation.1
Fig.1 Examples of glycosylation use/potential use as markers in relation to stem cells and their differentiation.1

