Anti-Herpesvirus Glycan Antibody Development Service
As a leading authority in viral immunology and glycan analysis, Creative Biolabs offers specialized support for basic and preclinical infectious disease research through our advanced Anti-Viral Glycan Shield Antibody Development platform. The envelope glycoproteins of the Herpesviridae family, including Herpes Simplex Virus (HSV) and Cytomegalovirus (CMV), are heavily decorated with complex N-linked and O-linked glycans. These carbohydrate structures form a "viral glycan shield" that protects the virus from neutralizing antibodies while simultaneously mediating critical interactions with host cell receptors during viral entry. We provide a comprehensive custom antibody service designed to target these intricate glycoprotein complexes, enabling researchers to investigate glycan-dependent immune evasion mechanisms and identify potential targets for antiviral intervention in experimental settings.
The Role of Glycans in Herpesvirus Biology
The Herpesviridae family comprises large, enveloped DNA viruses that establish lifelong latent infections in their hosts. Key members such as HSV-1, HSV-2, CMV, Epstein-Barr Virus (EBV), and Varicella-Zoster Virus (VZV) utilize a conserved core fusion machinery composed of glycoproteins gB and the gH/gL complex. These fusion proteins, along with receptor-binding proteins like gD (in HSV) or gp350 (in EBV), are densely glycosylated. This glycosylation is not merely structural; it is functional. The viral glycan shield modulates the conformation of these fusion proteins, shielding conserved epitopes from the host immune system.
Furthermore, specific glycoforms on these viral proteins act as ligands for host lectins (e.g., DC-SIGN) or modify the affinity for cellular receptors such as nectin-1 or HVEM. Research indicates that the heterogeneity of the glycan shield—ranging from high-mannose to complex sialylated structures—plays a pivotal role in viral tropism, dictating whether the virus infects epithelial cells, neurons, or leukocytes. Understanding and targeting these specific glycan structures is essential for dissecting the mechanisms of viral entry and for the rational design of next-generation vaccines and monoclonal antibody therapies.
Pain Points in Anti-Herpesvirus Antibody Discovery
Developing high-affinity antibodies against herpesvirus glycoproteins presents unique challenges that often hinder research progress:
Host Mimicry and Tolerance
Herpesviruses utilize the host's own glycosylation machinery, resulting in a viral glycan shield that closely resembles "self" antigens. This similarity induces immune tolerance, making it extremely difficult to elicit a robust immune response against the glycan moieties using standard immunization protocols.
Structural Heterogeneity
The glycosylation of viral proteins such as gB or gD is highly heterogeneous and cell-type dependent. A virus produced in epithelial cells may display a different glycan profile than one produced in neurons. Standard antibodies often fail to recognize these subtle but functionally critical variations.
Epitope Masking
The dense array of N-linked glycans can physically mask underlying peptide epitopes. Generating antibodies that can either bind the glycan itself or penetrate this shield to bind the protein core requires precise antigen design and sophisticated screening strategies.
Conformational Instability
Viral glycoproteins like gB are metastable prefusion class III fusionogens. Their conformation—and thus their antigenic landscape—can change upon isolation. Antibodies raised against recombinant proteins may not recognize the native trimer on the virion surface.
Our Solutions: Anti-Herpesvirus Glycan Antibody Development
To overcome these barriers, Creative Biolabs utilizes a proprietary suite of glyco-engineering and antibody discovery technologies. We offer a fully custom antibody service tailored to the specific needs of herpesvirus research. Our platform integrates advanced antigen design, diverse host species immunization, and high-throughput screening to isolate rare clones that recognize specific glyco-epitopes or glycan-dependent conformations.
Target Identification and Antigen Design
We specialize in the expression of viral glycoproteins (gB, gD, gH/gL, gp350) in mammalian systems that replicate the native viral glycosylation patterns. We can engineer antigens with defined glycan structures (e.g., high-mannose vs. complex) to direct the immune response toward specific epitopes.
Custom Antibody Generation
Utilizing both hybridoma technology and phage display libraries, we generate high-affinity monoclonal antibodies. Our phage display platform allows for the selection of binders against toxic viral proteins and the isolation of antibodies that bind to conserved epitopes masked by the glycan shield.
Glycan-Specific Screening and Validation
We employ a rigorous screening process using glycan arrays and counter-selection strategies to ensure specificity. Our validation pipeline includes neutralization assays, ELISA, and flow cytometry to confirm that the antibodies recognize the native virion and block viral entry or cell-to-cell spread.
Antibody Engineering for Research Applications
For advanced studies, we offer antibody engineering services such as humanization, affinity maturation, and Fc modification to support mechanistic investigations of antiviral activity in vitro or in preclinical models. All reagents are provided for research use only, not for diagnostic or therapeutic purposes.
Project Workflow
Core Advantages
Authentic Glycosylation
Our expression systems preserve the complex native glycan structures found on viral envelopes.
High Affinity
We consistently achieve nanomolar or sub-nanomolar affinity for challenging viral targets.
Data-Driven
Every antibody comes with comprehensive validation data including SPR kinetics and epitope binning.
Broad Application
Suitable for basic virology, vaccine design, diagnostic development, and therapeutic research.
Inquire About Herpesvirus Antibodies
Published Data
Recent research has fundamentally shifted the paradigm in antiviral antibody discovery, particularly for complex targets like herpes simplex virus (HSV). A groundbreaking study focused on isolating monoclonal antibodies against HSV-2 glycoprotein B (gB) from vaccinated subjects yielded surprising results. The investigation characterized a potent antibody clone that, despite lacking traditional in vitro neutralizing activity—often the primary selection criterion in drug discovery—provided robust protection against lethal viral challenge in vivo. Mechanism of action studies revealed that this protection was not achieved by blocking viral entry, but rather through Fc gamma receptor (FcγR) activation, specifically driving Antibody-Dependent Cellular Cytotoxicity (ADCC). Structural analysis further mapped the antibody's epitope to domain IV of gB, a region distinct from those targeted by neutralizing binders. This finding is pivotal for antibody development strategies, demonstrating that the viral glycan shield and conformational masking can be circumvented by leveraging Fc-mediated effector functions. It underscores the critical need for comprehensive screening platforms that evaluate candidates beyond simple neutralization, incorporating ADCC and Antibody-Dependent Cellular Phagocytosis (ADCP) assays to capture the full therapeutic potential of anti-glycan and anti-glycoprotein antibodies for infectious disease treatment.
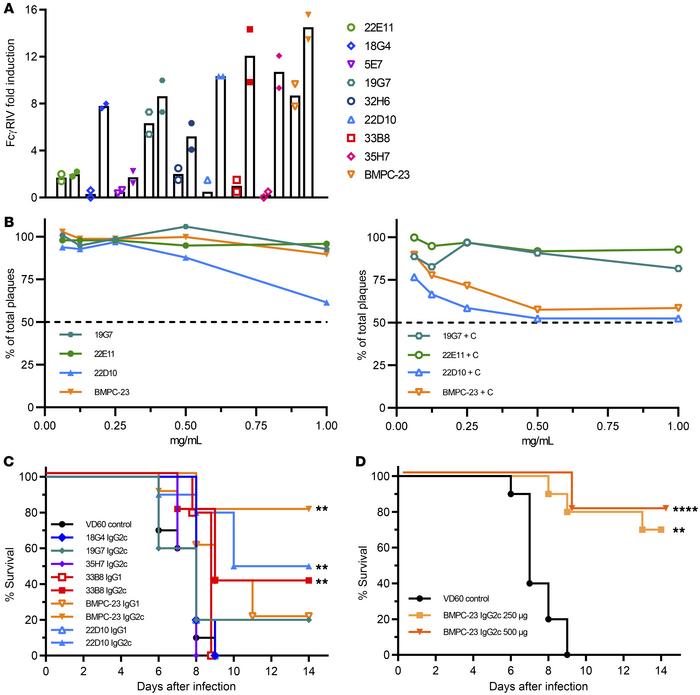 Fig.1 In vivo protective efficacy of non-neutralizing anti-gB monoclonal antibody mediated by Fc-dependent effector functions.1
Fig.1 In vivo protective efficacy of non-neutralizing anti-gB monoclonal antibody mediated by Fc-dependent effector functions.1
FAQs
Why is the viral glycan shield important for antibody development?
The viral glycan shield acts as a physical barrier that masks conserved protein epitopes from the host immune system. However, it also presents unique, virus-specific glycan epitopes. Antibodies that can penetrate this shield or bind specifically to the viral glycans themselves can be potent neutralizers, making them high-value targets for antiviral drug development.
Which herpesvirus glycoproteins do you target?
We primarily target the major envelope glycoproteins involved in viral entry and fusion. This includes gB, gD, and the gH/gL complex for HSV-1, HSV-2, VZV, and CMV. We also work with EBV gp350 and gH/gL/gp42 complexes. If you have a specific target of interest, we can design a custom campaign.
Can you generate antibodies that distinguish between HSV-1 and HSV-2?
Yes. While gB is highly conserved between HSV-1 and HSV-2, gD and other envelope proteins exhibit significant divergence. Through our negative selection and counter-screening protocols, we can isolate type-specific antibodies that recognize unique epitopes or glycoforms present on only one serotype.
What host species are available for immunization?
We offer immunization in mice, rats, rabbits, and llamas (for VHH production). Rabbits are particularly useful for generating high-affinity antibodies against glycosylated antigens due to their unique immune repertoire.
Do you offer validation services for viral neutralization?
Yes, we can perform in vitro virus neutralization assays (plaque reduction assays) using relevant cell lines. We also offer epitope mapping and kinetic characterization (SPR/BLI) to fully characterize the mechanism of action of your custom antibody.
How long does a custom project take?
Timelines vary based on the complexity of the antigen and the chosen discovery platform. A standard hybridoma project typically takes 4-6 months, while phage display projects can often be completed in 3-4 months. We provide a detailed timeline upon project initiation.
What Our Customers Say
"Targeting the gB glycoprotein of CMV was a major hurdle for us due to its dense glycosylation. Creative Biolabs designed a strategy using mammalian-expressed antigens that yielded an antibody with exceptional neutralizing potency. Their expertise in the viral glycan shield is evident."
"We needed a specific monoclonal antibody for HSV-2 gD that would not cross-react with HSV-1. The team delivered a clone with excellent type-specificity. The validation data they provided was comprehensive and saved us months of internal testing."
"The custom antibody service provided by Creative Biolabs is top-tier. We appreciated the regular updates and scientific insights provided during the project. The anti-glycan antibodies are working perfectly in our entry inhibition assays."
"Excellent support for our antiviral drug discovery program. The antibodies we received against VZV gE have high affinity and specificity. Highly recommended for complex viral glycoprotein targets."
Reference:
- Kuraoka, M., et al. "A non-neutralizing glycoprotein B monoclonal antibody protects against herpes simplex virus disease in mice." Journal of Clinical Investigation 133.3 (2023): e161968. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1172/JCI161968
