Anti-Plant & Algal Glycan Antibody Development Service
The cell walls of plants and algae represent some of the most intricate and biologically significant structures in the natural world. Composed of a highly dynamic and heterogeneous network of plant glycans, including polysaccharides and glycoproteins, these extracellular matrices are fundamental to plant growth, morphogenesis, structural integrity, and defense against biotic and abiotic stresses. Understanding the precise micro-architecture of the cell wall—how polymers like cellulose, hemicellulose, and pectin are synthesized, modified, and cross-linked—is crucial for advancing fields ranging from fundamental plant biology to biomass utilization and biofuel production.
At Creative Biolabs, we recognize that the complexity of these glycan structures poses a significant analytical challenge. Conventional chemical analyses, while useful for determining overall composition, often fail to capture the spatial distribution and subtle structural modifications of glycans within the tissue context. To bridge this gap, we offer a comprehensive anti-plant and algal glycan antibody development service. Our platform is dedicated to generating high-affinity, high-specificity monoclonal antibodies that serve as powerful molecular probes. These tools enable researchers to dissect the cell wall with sub-cellular resolution, distinguishing between fine structural nuances such as the degree of methyl-esterification in homogalacturonan or specific side-chain substitutions in hemicelluloses.
Unlocking the Secrets of Plant and Algal Cell Wall Architecture
The plant cell wall is a sophisticated composite material, primarily consisting of crystalline cellulose microfibrils embedded in a hydrated matrix of hemicelluloses (such as xyloglucan, xylan, and mannan), pectins, and structural proteins. This matrix is not static; it undergoes continuous remodeling during cell division, expansion, and differentiation. For instance, the modification of pectin by pectin methylesterases (PMEs) alters the mechanical properties of the wall, influencing cell adhesion and expansion rates. Similarly, the substitution patterns of hemicelluloses can dictate their interaction with cellulose, thereby modulating wall stiffness.
Algal cell walls, particularly those of marine macroalgae (seaweeds), present an even greater diversity of glycan structures. They often contain unique sulfated polysaccharides, such as fucoidan in brown algae and carrageenan in red algae, which have distinct physicochemical properties and immense industrial potential in food, pharmaceuticals, and biomaterials. The ability to specifically detect and quantify these polymers is essential for quality control and for understanding the evolutionary biology of cell walls.
Despite their importance, glycans are inherently difficult to study due to their non-template-driven biosynthesis and structural heterogeneity. A plant cell wall antibody functions as a molecular lens, allowing scientists to visualize specific epitopes in situ via immunofluorescence microscopy or electron microscopy, effectively mapping the glycome of the plant or algal cell.
Challenges in Developing Anti-Glycan Antibodies
Generating robust antibodies against carbohydrate targets involves overcoming several immunological and technical hurdles that are distinct from protein antibody production:
- T-Cell Independence: Most polysaccharides are T-cell independent antigens. When injected alone, they typically elicit a weak immune response dominated by low-affinity IgM antibodies and fail to induce immunological memory.
- Structural Similarity & Cross-Reactivity: Many cell wall glycans share common structural motifs (e.g., backbone linkages). An antibody intended for a specific epitope may cross-react with similar structures on different polymers, leading to ambiguous results. For example, distinguishing between a xyloglucan antibody target and a related glucan backbone requires precise epitope definition.
- Context-Dependent Binding: The accessibility of a glycan epitope can be masked by other cell wall components (e.g., pectin masking xyloglucan), making it critical to screen antibodies in relevant biological contexts.
Comprehensive Development Workflow
Start Your Custom Project
Specialized Antibody Development Services
Our service portfolio covers the entire spectrum of plant and algal cell wall polysaccharides. We can generate antibodies with specificities analogous to the widely used JIM, LM, and CCRC series, or develop completely novel binders for unique or newly discovered structures.
Anti-Pectin Antibody Development
Pectins are arguably the most complex class of cell wall polysaccharides, playing key roles in cell adhesion (middle lamella), expansion, and signaling. We develop antibodies against all three major pectic domains: Homogalacturonan (HG), Rhamnogalacturonan-I (RG-I), and Rhamnogalacturonan-II (RG-II).
- Targeting Homogalacturonan Methylation: The degree of methyl-esterification (DM) of HG is a critical regulator of cell wall mechanics. We can generate clones with specificities similar to the JIM5 antibody (which binds to low-esterified HG, often associated with stiff, calcium-crosslinked gels) and the JIM7 antibody (which binds to high-esterified HG, typical of fluid, growing walls). We also develop antibodies like the LM series antibody (e.g., LM19, LM20) to provide a gradient of DM recognition tools.
- Targeting RG-I Side Chains: We generate antibodies against the diverse side chains of RG-I, including linear and branched arabinans and galactans, which are often developmentally regulated.
- General Pectin Tools: Our broad-spectrum pectin antibody options serve as excellent markers for monitoring overall pectin integrity and extraction efficiency during biomass processing.
Anti-Hemicellulose Antibody Development
Hemicelluloses tether cellulose microfibrils, forming a load-bearing network. Antibodies against these polymers are essential for studying cell wall extensibility and for identifying specific hemicellulose populations in different tissue types.
- Xyloglucan Specificity: Xyloglucan is the major hemicellulose in dicots. We offer development of antibodies targeting specific oligomeric motifs (e.g., XXXG vs. XXLG) to distinguish between species or tissue-specific isoforms. We also specialize in fucosylated xyloglucan antibody production (similar to CCRC-M1), crucial for studying the role of fucosylation in cell wall loosening.
- Xylan Profiling: For research on secondary cell walls and biomass crops (grasses, poplars), we develop xylan antibody tools that recognize specific substitution patterns, such as arabinosylation or glucuronosylation (equivalent to LM10, LM11), helping to map xylan heterogeneity.
- Mannan Detection: Our mannan antibody plant services provide reagents for detecting glucomannans and galactomannans, which are abundant in seeds and gymnosperm woods.
Anti-Proteoglycan & Structural Protein Antibody
The cell wall also contains structural proteins and proteoglycans that function in signaling, development, and defense.
- AGP Epitopes: Arabinogalactan proteins (AGPs) are heavily glycosylated proteoglycans implicated in diverse processes from somatic embryogenesis to pollen tube growth. We develop arabinogalactan protein antibody clones (similar to JIM13, LM2) targeting the complex glycan epitopes on these molecules.
- Extensin Targets: We produce extensin antibody tools (like JIM11, JIM20) that recognize the hydroxyproline-rich glycoproteins (HRGPs) that form a reinforcing scaffold in the cell wall, particularly in response to pathogen attack or wounding.
Anti-Algal Glycan Antibody Development
Algal cell walls are rich in unique polysaccharides that are not found in land plants. We are pioneers in developing reagents for marine biotechnology applications.
- General Algal Profiling: Our algal glycan antibody services offer broad capabilities for screening microalgae and macroalgae populations.
- Alginate Block Structures: We can generate alginate antibody clones capable of distinguishing between mannuronate (M) blocks, guluronate (G) blocks, and alternating MG blocks, which determine the gelling properties of the polymer.
- Carrageenan Types: We develop carrageenan antibody tools with specificity for Kappa, Iota, or Lambda carrageenan types, aiding in the quality control of red algal extracts.
- Fucoidan Complexity: Our fucoidan antibody development targets the sulfated fucans of brown algae, supporting research into their anticoagulant and anti-inflammatory properties.
Anti-Cellulose Antibody Development
Developing a true cellulose antibody is exceptionally challenging due to the crystalline and insoluble nature of the target. To overcome this, we utilize an innovative approach involving specific Carbohydrate-Binding Modules (CBMs) fused to antibody domains (CBM-mAbs). This technology allows us to generate probes that can distinguish between amorphous and crystalline regions of cellulose microfibrils, providing critical insights for biofuel research and the study of enzymatic hydrolysis efficiency.
Why Choose Creative Biolabs?
Unmatched Epitope Precision
Our screening process allows us to distinguish between subtle differences, such as methylation and acetylation patterns in pectin and hemicellulose.
Comprehensive Validation Arrays
We validate every clone using extensive glycan arrays containing over 300 defined plant and algal cell wall fragments to ensure specificity.
Application-Ready Reagents
Our antibodies are tested and optimized for immediate use in Immunofluorescence (IF), ELISA, and immunogold labeling protocols.
Custom Conjugation Services
We offer direct conjugation to biotin, FITC, or HRP, enabling diverse imaging and detection applications tailored to your lab's needs.
How to Start Your Custom Project
Starting a project with Creative Biolabs is simple and collaborative. Our team acts as an extension of your laboratory, providing expert consultation to ensure the final antibody meets your specific research needs.
Step 1: Inquiry & Needs Assessment
Submit your inquiry online or via email. Tell us about your target glycan and your intended application. Our technical specialists will review your requirements to understand the biological context.
Step 2: Strategy Design & Proposal
Based on your goals, our PhD-level scientists will design a tailored development strategy. We will propose the optimal immunogen (neoglycoprotein or cell wall fraction), host species, and screening algorithm. You will receive a detailed project proposal outlining the timeline, milestones, and costs for your approval.
Step 3: Order Confirmation & Initiation
Once the proposal is accepted, we formalize the order and immediately initiate the project. You will be assigned a dedicated project manager who will provide regular updates on immunization progress, titer checks, and screening results.
Step 4: Delivery & Support
Upon completion, we deliver the purified antibodies along with a comprehensive validation report. Our support continues after delivery, assisting you with protocol optimization for your specific assays.
Published Data
Monoclonal antibodies are indispensable tools for visualizing the dynamic changes in cell wall composition during plant development. A relevant study utilized a panel of anti-pectin antibodies to map the distribution of homogalacturonan (HG) epitopes during the formation of vascular tissues in Poplar (Populus species).
The researchers employed JIM5 (specific for low-methylesterified HG) and JIM7 (specific for high-methylesterified HG) to dissect the chemical remodeling of the cell wall in the procambium-cambium continuum. The study demonstrated distinct spatial patterns: JIM5 labeling was intense in the cell corners and middle lamella of the phloem and developing xylem, suggesting a role for calcium-crosslinked acidic pectin in cell adhesion. In contrast, JIM7 labeling was more uniform across the cell walls of dividing cambial cells, indicating the secretion of highly esterified pectin during active growth.
These findings, enabled by the specificity of the JIM series antibodies, provided crucial insights into how differential pectin methylation regulates the mechanical properties of wood fibers. This case exemplifies how specific anti-glycan antibodies allow researchers to track subtle chemical modifications in situ that are invisible to standard staining techniques.
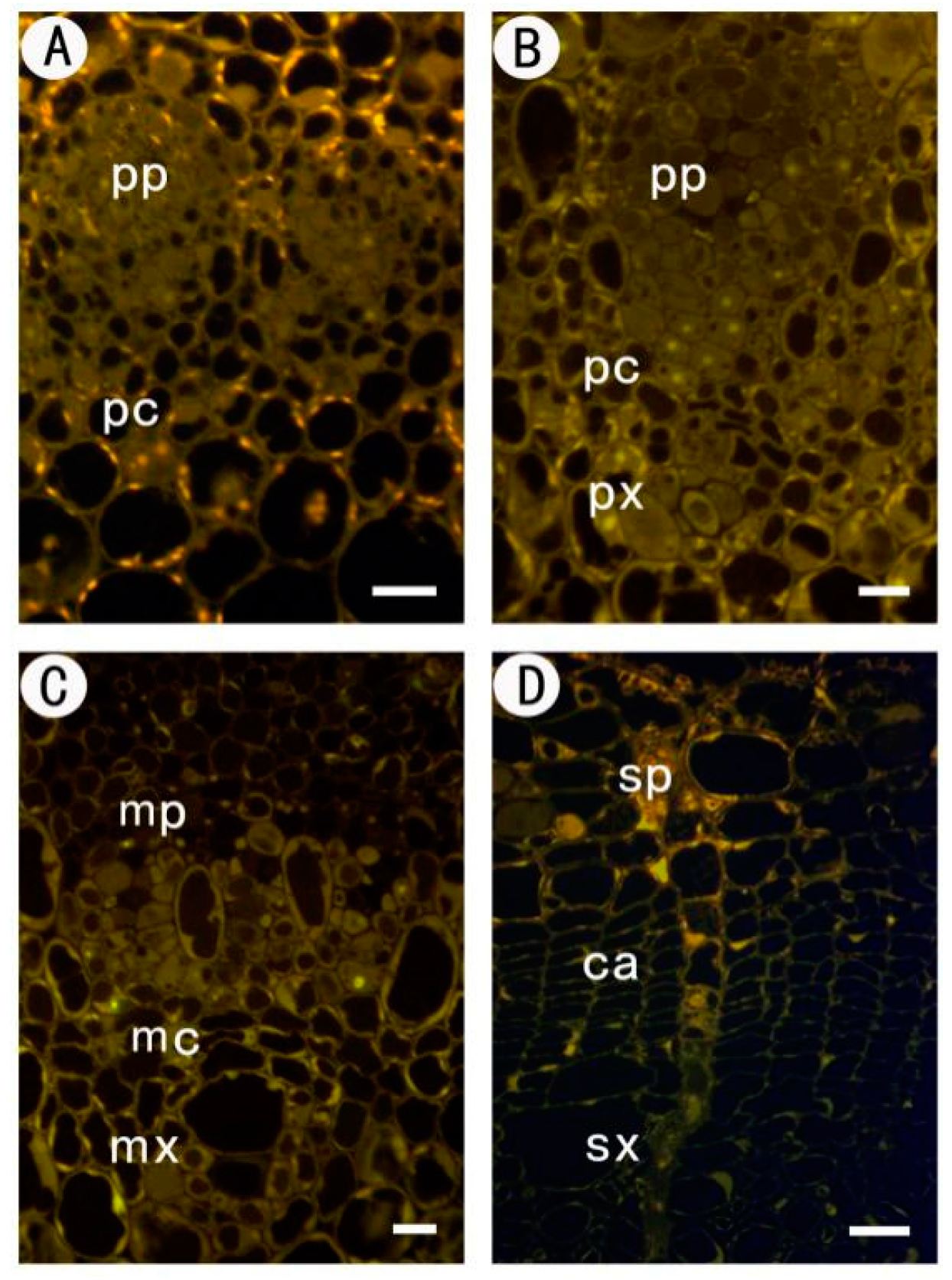 Fig.1 Immunofluorescence localization of JIM5 and JIM7 pectin epitopes in poplar stem cross-sections.1
Fig.1 Immunofluorescence localization of JIM5 and JIM7 pectin epitopes in poplar stem cross-sections.1
FAQs
Do your antibodies cross-react between different plant species?
Generally, yes. The fundamental glycan structures of the primary cell wall (e.g., the homogalacturonan backbone, the xyloglucan backbone) are highly conserved across the plant kingdom, particularly among angiosperms. An antibody raised against pectin from one species (e.g., tobacco) will typically recognize the same epitope in others (e.g., Arabidopsis, tomato), provided the specific structural features (like methyl-esterification or substitution patterns) are present. However, for more specialized structures found in specific families (like mixed-linkage glucans in grasses), species-specific validation is recommended.
Can you develop antibodies against specific sulfated algal polysaccharides?
Yes, absolutely. We have specific experience with the complex polysaccharides found in marine algae. We can generate antibodies against specific substructures of fucoidan (sulfated fucans), carrageenan (distinguishing Kappa, Iota, Lambda forms), and ulvan. These antibodies are crucial for distinguishing between different algal species and for characterizing the composition of industrial algal extracts.
What is the functional difference between JIM5 and JIM7 antibodies?
JIM5 and JIM7 are both monoclonal antibodies that target homogalacturonan (HG), but they recognize different patterns of methyl-esterification, which correlate with different physical properties of the cell wall. JIM5 preferentially binds to HG with a low degree of methyl-esterification (low-DE). Low-DE pectin can be cross-linked by calcium ions to form stiff gels. In contrast, JIM7 binds to HG with a higher degree of methyl-esterification (high-DE), which is typical of newly secreted pectin in looser, growing walls. Using both allows researchers to map the stiffness and maturity of the cell wall.
Are these antibodies suitable for electron microscopy (Immunogold labeling)?
Yes, many of our anti-glycan antibodies, including the LM and JIM series equivalents, work excellently in immunogold labeling for Transmission Electron Microscopy (TEM). This application allows for the ultrastructural localization of cell wall components, such as determining whether a glycan is located in the middle lamella, primary wall, or secondary wall. We can advise on optimal fixation and embedding protocols to preserve glycan antigenicity.
How do you ensure the antibody differentiates between Xylan and Xyloglucan?
Cross-reactivity between hemicelluloses is a common concern. We ensure specificity through our rigorous screening process using glycan microarrays. We test candidate clones against a library of pure, defined oligosaccharides. For a xyloglucan antibody, we select clones that bind to the glucose-xylose backbone motif (e.g., XXXG) but show no binding to xylan (xylose backbone) or other glucans. Similarly, xylan antibodies are screened to ensure they recognize the beta-1,4-xylose backbone or specific arabinose/glucuronic acid substitutions without binding to xyloglucan.
What is the typical timeframe for a custom anti-glycan antibody project?
A typical project, from immunogen preparation to the delivery of purified antibody, takes approximately 4 to 6 months. This timeline includes the complex steps of immunizing animals (which requires multiple boosts to elicit a response against carbohydrate antigens), fusion and hybridoma generation, and the extensive multi-step screening process required to identify clones with the high specificity needed for cell wall research. We provide regular updates and interim reports throughout the process.
Reference:
- Ma, L., et al. "Dynamic Changes of Pectin Epitopes in Cell Walls during the Development of the Procambium–Cambium Continuum in Poplar." International Journal of Molecular Sciences 18.8 (2017): 1716. Distributed under Creative Commons Attribution 4.0 International License (CC BY 4.0). https://doi.org/10.3390/ijms18081716
