The Clinical Rise of Glycosphingolipids: Targeting GD2, Globo H, and GM3
For decades, cancer immunotherapy has fixated on protein targets. Yet the surface of every cell is cloaked in a dense forest of sugars—the glycocalyx. Hidden within this dense carbohydrate layer lies a class of underappreciated but potent targets that are now transforming the treatment of solid tumors: glycosphingolipids (GSLs). Unlike glycoproteins—where carbohydrates are attached to a protein scaffold—glycosphingolipids consist of a hydrophilic glycan moiety linked to a lipid anchor embedded in the cell membrane. In cancer, the biosynthesis of these molecules is frequently dysregulated, leading to tumor-selective overexpression of specific structures such as GD2, Globo H, and the Neu5Gc-containing variant of GM3. Far from being inert markers, these glycans actively drive tumor progression, immune evasion, and metastasis.
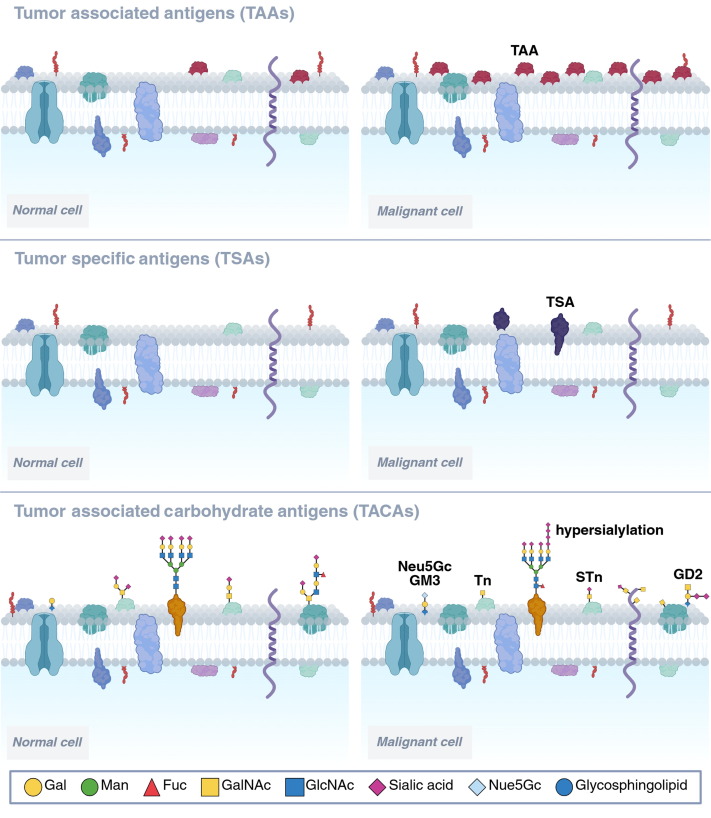 Fig.1 Comparison of tumor antigens: TAAs, TSAs, and TACAs.1
Fig.1 Comparison of tumor antigens: TAAs, TSAs, and TACAs.1
Developing antibodies against these membrane-anchored sugars presents unique challenges, as their structures are compact, often poorly immunogenic, and difficult to display in a native-like conformation. Yet recent clinical breakthroughs prove that, with the right binder, glycolipid-targeted therapies can be life-saving. At Creative Biolabs, we specialize in overcoming the hydrophobic and conformational hurdles inherent to these targets, offering end-to-end solutions for anti-glycolipid antibody discovery and engineering.
GD2: The Benchmark of Glycolipid Targets
Among all tumor-associated carbohydrate antigens (TACAs), the ganglioside GD2—a disialoganglioside—stands out as the most clinically validated glycolipid target. It is highly overexpressed in nearly all cases of high-risk pediatric neuroblastoma, while remaining largely absent from healthy tissues except peripheral nerves. This near-tumor-specific expression makes GD2 an ideal target for immunotherapy.
Dinutuximab
Dinutuximab, a chimeric IgG1 monoclonal antibody, was the first anti-GD2 therapy approved by the FDA.
- Clinical Impact: In combination with GM-CSF, IL-2, and isotretinoin, it significantly improves both event-free survival (EFS) and overall survival (OS) in high-risk neuroblastoma.
- Key Limitation: GD2 is expressed at low levels on peripheral nerves, leading to severe neuropathic pain—a significant dose-limiting toxicity. Slow, prolonged infusions are required to manage infusion-related reactions.
Naxitamab
The next-generation Naxitamab is a humanized IgG1 monoclonal antibody approved for the treatment of relapsed/refractory neuroblastoma.
- Advantage: While sharing a similar safety profile (including neuropathic pain), its optimized binding kinetics enable rapid infusion, dramatically improving patient comfort and clinic workflow.
Expanding Horizons: GD2 CAR-T for Brain Tumors
Perhaps most exciting is GD2's emergence as a target in diffuse midline glioma (DMG)—a lethal pediatric brain tumor with no effective standard therapy. A Phase I trial tested autologous CAR-T cells engineered with an anti-GD2 scFv (derived from the murine 14G2a clone, subsequently humanized). Remarkably:
- 9 of 11 patients showed neurological improvement.
- 7 patients experienced tumor shrinkage, with some responses described as pronounced.
To mitigate CNS toxicity, CAR-T cells were equipped with a safety switch (RQR8), allowing rapid depletion if needed. This success demonstrates that cellular therapies can safely and effectively target glycolipids like GD2—provided the antibody binder is exquisitely specific, for researchers developing next-generation CAR constructs, access to high-affinity, low-cross-reactivity anti-ganglioside binders is essential.
Globo H: The Rising Star in Epithelial Cancers
While GD2 dominates in neuroectodermal tumors, Globo H—a hexasaccharide-containing glycosphingolipid—is emerging as a premier target in breast, gastric, and pancreatic cancers. Unlike protein antigens, which can mutate, Globo H is defined by its invariant carbohydrate structure, requiring antibodies that recognize precise sugar linkages without cross-reacting with healthy glycans. OBI Pharma is leading clinical development with two complementary strategies:
OBI-888
This humanized IgG1 mAb induces antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). In a Phase I/II trial (n=54), it showed a favorable safety profile up to 20 mg/kg with no maximum tolerated dose (MTD) reached. 20–28% of patients achieved stable disease, proving that glycolipid targeting can be both safe and biologically active in epithelial cancers.
OBI-999
To enhance potency against bulky tumors, OBI-999 conjugates the same anti-Globo H antibody to monomethyl auristatin E (MMAE). In Phase I (n=15), five patients achieved stable disease, with one maintaining response for over 13 cycles. The MTD was 1.2 mg/kg, with neutropenia as the dose-limiting toxicity. The program has now advanced to Phase II with mandatory Globo H biomarker selection, underscoring the importance of patient stratification in TACA-directed therapy.
Neu5Gc-GM3: A Xenogenic Antigen in Human Tumors
Another intriguing target is Neu5Gc-GM3—a variant of the simple ganglioside GM3 that carries N-glycolylneuraminic acid (Neu5Gc) instead of the common N-acetyl form (Neu5Ac). Crucially, humans lack the enzyme to synthesize Neu5Gc due to an evolutionary gene deletion. However, dietary Neu5Gc (found in red meat and dairy) can be metabolically incorporated into the membranes of tumor cells, creating a xenogenic antigen on human cancer cells. This makes Neu5Gc-GM3 a highly tumor-selective target in theory.
Although clinical development remains in its early stages, researchers have isolated human scFv antibodies against Neu5Gc-GM3 from phage libraries derived from cancer survivors. The key challenge lies in discriminating between Neu5Gc and Neu5Ac—a single oxygen atom difference that demands atomic-level precision from antibodies.
Why Are Glycolipids So Hard to Target?
Despite promising clinical data, glycolipids remain underexplored compared to proteins. Three core challenges explain why:
Amphipathic Nature
Their lipid tails embed in the membrane while glycans extend outward, making it difficult to purify or present them in native conformation for immunization or screening.
Structural Homology
Minor differences—such as an α2,3 vs. α2,6 sialic acid linkage—can separate a tumor antigen from a healthy self-glycan.
T-Cell Independence
Carbohydrates typically elicit weak, non-memory B-cell responses, which complicates the discovery of antibodies.
Overcoming these barriers requires more than conventional hybridoma technology. Successful programs now leverage glycoengineered cell lines that stably display native GSLs, immune libraries from convalescent donors, and advanced display platforms to isolate rare, high-specificity binders.
Unlocking the Next Generation of Glycolipid Therapies
The clinical validation of GD2 and Globo H confirms that glycosphingolipids are no longer niche curiosities—they are high-value, druggable targets for cancers resistant to protein-directed therapies. The next wave—targeting GM3, GD3, SSEA-3/4, or complex globosides—will demand antibodies with unprecedented specificity and affinity. At Creative Biolabs, we provide a specialized platform to navigate this complex landscape.
Native-like Glycolipid Antigen Design
Using liposomal or membrane-display systems to ensure correct presentation of the target antigen.
High-Diversity Phage Display Libraries
Generated from immunized or survivor-derived repertoires to isolate rare binders.
Glycan Microarray Screening
Cross-reactivity screening to validate a novel glycolipid target and ensure specificity.
End-to-End Antibody Engineering
Optimization for CAR-T, ADC, or naked antibody formats to suit your therapeutic goals.
Whether you're validating a novel glycolipid target or advancing a preclinical therapeutic candidate, our team is equipped to help you turn the tumor glycocalyx into a therapeutic vulnerability. Ready to explore glycolipid-targeted therapy?
Contact Creative Biolabs Today
FAQs
What is the main structural difference between glycoproteins and glycosphingolipids?
The key difference lies in the anchor. In glycoproteins, the carbohydrate chain is covalently attached to amino acid residues on a protein backbone. In glycosphingolipids (GSLs), the glycan head group is linked directly to a lipid ceramide tail embedded in the cell membrane. This unique amphipathic structure requires specialized strategies for antigen isolation and antibody development.
Why are Tumor-Associated Carbohydrate Antigens (TACAs) often considered poor immunogens?
TACAs, including glycolipids such as GD2 and Globo H, are generally T-cell-independent antigens. Unlike proteins, they do not naturally recruit helper T cells to stimulate a robust, long-lasting memory B cell response. This makes generating high-affinity IgG antibodies challenging, often requiring specialized conjugation techniques or immune libraries from cancer survivors to succeed.
Is GD2 expression exclusive to cancer cells?
GD2 is highly restricted but not 100% exclusive. It is virtually absent from most healthy tissues but is expressed at low levels on peripheral nerves and central nervous system tissues. This limited expression profile is what makes it a potent target for neuroblastoma therapy, though it also necessitates careful management of on-target side effects like neuropathic pain.
Can glycosphingolipids be targeted by CAR-T cells?
Yes. Recent breakthroughs have demonstrated that CAR-T cells can effectively target glycolipids. For example, CAR-T therapies targeting the ganglioside GD2 have shown significant promise in treating diffuse midline glioma (DMG). By engineering the CAR with a specific antibody fragment (scFv), T-cells can recognize the lipid-anchored sugar and eliminate the tumor cell.
Is glycolipid expression consistent across all tumor cells?
Not always. Tumor heterogeneity poses a significant challenge, as expression levels can vary widely between patients or even within a single tumor lesion. This variability highlights the importance of biomarker screening and supports the use of potent modalities like ADCs to overcome potential antigen escape.
Are glycosphingolipids suitable targets for Antibody-Drug Conjugates (ADCs)?
Yes, provided the antibody internalizes upon binding. Clinical candidates, such as OBI-999 (targeting Globo H) and ST1 (targeting sTn), have demonstrated that antibodies targeting carbohydrate antigens can effectively deliver potent cytotoxic payloads, including MMAE, directly into tumor cells, inducing regression.
Reference:
- Meier, Edward PW, and Andreas H. Laustsen. "Advances in antibody-based strategies for targeting cancer-associated glycopeptide antigens." Drug Discovery Today (2025): 104507. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1016/j.drudis.2025.104507
