Keratan Sulfate: A Key Player in Corneal Transparency and Bone Health
Keratan sulfate (KS) is a highly specialized glycosaminoglycan (GAG) that is distinguished by its unique polylactosamine backbone and sulfation patterns. Unlike other GAGs that contain uronic acids, KS consists of repeating disaccharide units of galactose and N-acetylglucosamine. This structural distinction underpins its specific physiological roles, particularly in the maintenance of tissue hydration, structural integrity, and cell signaling. At Creative Biolabs, we offer comprehensive services to advance the study of this complex molecule, including our Anti-Keratan Sulfate Antibody Development service, which provides researchers with precise tools for biomarker discovery and histological analysis.
Structural Diversity: The Three Classes of Keratan Sulfate
The functional versatility of KS arises from its heterogeneous structure. It is classified into three major types based on its linkage to the core protein, a feature that dictates its tissue distribution and biological function:
KS-I (Corneal KS)
Linked via an N-glycosidic bond to asparagine residues. This type is predominantly found in the cornea, where it is covalently bound to small leucine-rich proteoglycans (SLRPs) such as lumican, keratocan, and mimecan. Its high degree of sulfation is critical for corneal transparency.
KS-II (Skeletal KS)
Linked via an O-glycosidic bond to serine or threonine residues. KS-II is primarily abundant in cartilage, where it attaches to the large aggregating proteoglycan, aggrecan. It contributes significantly to the osmotic properties and compressive stiffness of cartilage.
KS-III (Brain KS)
Linked via an O-glycosidic bond to mannose. Found in specific neural tissues, KS-III is involved in microglial regulation and neural plasticity. Its expression is tightly regulated during development and in response to injury.
The Mechanism of Corneal Transparency
The cornea's ability to transmit light with minimal scattering is a marvel of biological engineering, and keratan sulfate cornea interactions are central to this phenotype. The corneal stroma consists of strictly organized collagen fibrils. KS-proteoglycans (KSPGs) bind to these fibrils at specific intervals, regulating their diameter and ensuring uniform interfibrillar spacing.
The Lattice Theory of Transparency
Transparency depends on the destructive interference of scattered light, which requires the collagen fibrils to be arranged in a quasi-crystalline lattice. The highly sulfated KS chains extend into the interfibrillar space, creating a hydrophilic environment. The hydration forces and steric repulsion generated by these negative charges prevent the collagen fibrils from fusing or becoming disorganized. When KS synthesis is disrupted, as seen in certain pathologies, the fibrils aggregate irregularly, leading to light scattering and corneal opacity.
Function in Bone and Cartilage Homeostasis
In the skeletal system, KS acts as a critical shock absorber. Within bone cartilage, KS chains are attached to the aggrecan core protein alongside chondroitin sulfate. While chondroitin sulfate provides the bulk of the negative charge, KS plays a specialized role in tissue stabilization.
- Osmotic Pressure Regulation: The sulfate groups on KS attract cations and water molecules, generating internal osmotic pressure that resists compressive loads during movement.
- Protection from Proteolysis: The presence of KS chains can sterically hinder the access of proteolytic enzymes (such as aggrecanases) to the core protein, thereby slowing down matrix degradation.
- Cellular Interaction: KS motifs can interact with specific cell surface receptors, influencing chondrocyte behavior and matrix turnover rates.
Keratan Sulfate in Neural Plasticity and Repair
Beyond its structural functions in connective tissues, KS is increasingly recognized for its dynamic role in the central nervous system (CNS). In the brain and spinal cord, phosphacan and other proteoglycans carry KS chains that modulate neural plasticity. Unlike the supportive role in the cornea, CNS KS often acts as an inhibitory signal, limiting plasticity to stabilize neural circuits.
Glial Scar Formation
Following spinal cord injury (SCI) or traumatic brain injury, astrocytes and other glial cells become reactive and upregulate the secretion of KS-proteoglycans. These molecules accumulate in the glial scar, forming a chemical barrier. While this barrier seals off the injury site to prevent infection and further damage, it significantly inhibits axon regeneration.
Therapeutic Targeting
Because KS inhibits neurite outgrowth, enzymatic digestion of KS chains (using keratanase) or neutralization via specific anti-KS antibodies has shown promise in preclinical models of CNS injury. These interventions can lower the inhibitory threshold of the scar tissue, potentially creating a more permissive environment for nerve regeneration and functional recovery.
Clinical Relevance: KS as a Biomarker
Macular Corneal Dystrophy (MCD)
MCD is an autosomal recessive disorder characterized by the accumulation of non-sulfated KS in the corneal stroma. It is caused by mutations in the CHST6 gene, which encodes corneal N-acetylglucosamine-6-O-sulfotransferase. The resulting loss of sulfation disrupts the KSPG-mediated spacing of collagen fibrils, leading to progressive clouding of the cornea and severe visual impairment. This condition underscores the absolute necessity of KS sulfation for corneal transparency.
Osteoarthritis and Joint Disease
In osteoarthritis, the breakdown of cartilage matrix releases KS fragments into the synovial fluid and serum. Quantifying serum KS levels has emerged as a potential biomarker for monitoring cartilage turnover and disease progression. An accurately calibrated ks proteoglycan assay can help researchers assess the efficacy of chondroprotective therapies in preclinical models.
Our Solutions for KS Research
Investigating KS requires specialized tools due to its low immunogenicity and structural heterogeneity. Creative Biolabs provides a suite of services designed to overcome these challenges.
Anti-Keratan Sulfate Antibody Development
We generate high-affinity monoclonal antibodies (e.g., clones 5D4-like) and novel binders using phage display technology. Our antibodies are validated for specificity against sulfated motifs, ensuring no cross-reactivity with other GAGs.
Glycoarray Platforms
Screen your potential binders or investigate KS-protein interactions using our high-throughput glycan arrays, which feature defined KS oligosaccharide libraries.
Glycosylation Analysis
We offer comprehensive structural characterization of KS chains using mass spectrometry (LC-MS/MS) and HPLC to determine chain length, sulfation degree, and disaccharide composition.
Inquire About KS Research Tools
Published Data
The transparent nature of the cornea is not accidental but the result of a highly organized extracellular matrix, a phenomenon extensively analyzed in recent literature regarding ocular surface homeostasis. Central to this architecture is the interaction between collagen fibrils and small leucine-rich proteoglycans (SLRPs), specifically lumican and keratocan, which are heavily substituted with sulfated keratan sulfate (KS) chains. These KS side chains are highly anionic, allowing them to bind water and extend into the interfibrillar space. This extension creates a repulsive force that regulates the precise diameter and spacing of collagen fibrils, forming a "quasi-crystalline" lattice essential for light transmission. The critical nature of this mechanism is highlighted by pathological models; for instance, the loss of KS sulfation—mediated by enzymes like GlcNAc-6-O-sulfotransferase—leads to the aggregation of collagen fibrils and subsequent corneal opacity, a hallmark of Macular Corneal Dystrophy. Furthermore, immunohistochemical analysis reveals that KS is not randomly distributed but forms distinct patterns lining the stromal lamellae, distinguishing healthy tissue from pathological states. This data underscores the utility of sulfated KS not merely as a structural component, but as a sensitive biomarker for assessing corneal integrity and monitoring the progression of stromal dystrophies.
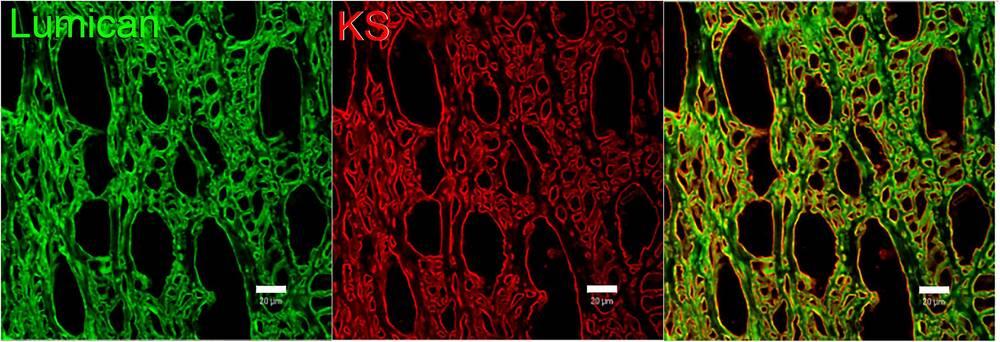 Fig.1 Spatial distribution of sulfated keratan sulfate and lumican in the human corneal stroma.1
Fig.1 Spatial distribution of sulfated keratan sulfate and lumican in the human corneal stroma.1
FAQs
What distinguishes KS-I from KS-II biochemically?
The primary difference lies in the linkage to the protein core. KS-I is N-linked to asparagine residues (typical of corneal proteoglycans), while KS-II is O-linked to serine or threonine residues (typical of skeletal proteoglycans). Additionally, their sulfation patterns and chain lengths can vary significantly between tissues.
Why is keratan sulfate critical for corneal transparency?
KS chains on proteoglycans like lumican regulate the diameter and spacing of collagen fibrils. They create a "quasi-crystalline" lattice that minimizes light scattering. Without sulfated KS, collagen fibrils aggregate irregularly, causing the cornea to become opaque.
Can KS levels be used as a biomarker for osteoarthritis?
Yes. During cartilage degeneration, aggrecan is cleaved, releasing KS-bearing fragments into the synovial fluid and blood. Elevated levels of antigenic KS in serum can serve as a marker for the rate of cartilage catabolism in osteoarthritis and rheumatoid arthritis.
What is the role of the CHST6 gene in KS function?
The CHST6 gene encodes corneal N-acetylglucosamine-6-O-sulfotransferase. This enzyme is responsible for adding sulfate groups to GlcNAc residues in corneal KS. Mutations in this gene prevent the sulfation of corneal KS, resulting in Macular Corneal Dystrophy.
What services does Creative Biolabs offer for KS research?
We offer custom antibody production (hybridoma and phage display), glycan array screening for specificity validation, and advanced analytical services like mass spectrometry to characterize KS chain structure and sulfation levels.
Reference:
- Puri, S., Coulson-Thomas, Y. M., Gesteira, T. F., & Coulson-Thomas, V. J. "Distribution and Function of Glycosaminoglycans and Proteoglycans in the Development, Homeostasis and Pathology of the Ocular Surface." Frontiers in Cell and Developmental Biology 8 (2020): 731. Distributed under Open Access license CC BY 4.0. https://doi.org/10.3389/fcell.2020.00731
