Anti-Hyaluronic Acid (HA) Antibody Development Service
Hyaluronic acid (HA), a non-sulfated glycosaminoglycan (GAG), is a critical component of the extracellular matrix (ECM) and plays pivotal roles in cell migration, proliferation, and differentiation. Its abnormal accumulation or degradation is frequently associated with pathological conditions, including cancer metastasis, inflammation, and fibrosis. However, generating a high-affinity anti-GAG antibody against HA is notoriously difficult due to its non-immunogenic nature and ubiquitous expression in healthy tissues. At Creative Biolabs, we overcome these hurdles by leveraging advanced phage display technology and proprietary antigen presentation strategies. We provide a comprehensive anti-hyaluronic acid (HA) antibody development service to deliver highly specific tools for oncology, wound healing, and diagnostic research.
Background: The Unique Challenge of Targeting a Non-Sulfated GAG
Unlike other GAGs such as chondroitin sulfate (CS), heparan sulfate (HS), or dermatan sulfate (DS), Hyaluronic Acid (HA) is unique in that it lacks sulfation. It is a simple linear polymer of repeating disaccharides (D-glucuronic acid and N-acetyl-D-glucosamine) synthesized at the plasma membrane by Hyaluronan Synthases (HAS1, HAS2, HAS3). This structural simplicity presents a dual challenge for antibody development:
- Lack of Charge-Based Epitopes: Most anti-GAG antibodies rely on specific sulfation patterns (e.g., 4-O-sulfation vs. 6-O-sulfation) to distinguish between glycan species. Since HA is non-sulfated, antibodies must recognize the specific conformational epitopes of the sugar backbone itself, or the secondary helical structures that form in longer chains.
- Extreme Conservation (Self-Antigen): The structure of HA is 100% conserved across all mammals. Consequently, the immune system of standard laboratory animals (mice, rabbits) is heavily tolerized to HA, making traditional immunization strategies ineffective.
Furthermore, HA exhibits distinct biological functions depending on its chain length. High Molecular Weight HA (HMW-HA, >1000 kDa) is generally anti-inflammatory and structural, while Low Molecular Weight HA (LMW-HA, <200 kDa) and HA oligosaccharides are potent signaling molecules that promote inflammation, angiogenesis, and tumor invasion via CD44 and RHAMM receptors. Developing an anti-hyaluronic acid antibody capable of distinguishing between these size variants or detecting HA in the presence of abundant sulfated GAGs requires a sophisticated, non-traditional approach.
Custom Anti-HA Antibody Development Services
Creative Biolabs utilizes a robust platform designed specifically for non-immunogenic, carbohydrate antigens. We do not rely on animal immunization. Instead, we use large-scale synthetic antibody libraries to isolate binders that recognize the specific 3D conformation of HA. Our service is modular and tailored to address the "size" and "specificity" challenges inherent to HA research.
Synthetic Phage Display Library Screening
To bypass immune tolerance, we employ synthetic human scFv and Fab phage display libraries. These libraries contain billions of antibody variants derived from non-immunized sources or synthetic designs. By panning against immobilized HA, we can select rare binders that recognize the non-sulfated HA backbone, which would never be generated in a living animal due to tolerance mechanisms.
Size-Specific Antigen Design (Oligosaccharide Engineering)
To generate antibodies that distinguish between LMW and HMW forms, we synthesize defined HA oligosaccharides (e.g., HA4, HA6, HA10, HA20). These haptens are conjugated to carrier proteins (BSA, KLH) or biotinylated with precise orientation. This forces the selection of antibodies that recognize the specific terminal structures or short-chain conformations characteristic of LMW-HA fragments often found in tumor microenvironments.
Specificity Profiling via Glycan Arrays
A critical step for HA antibodies is proving they do not bind sulfated GAGs. We use our high-throughput glycoarray platform to screen candidate clones against a comprehensive panel of GAGs (Chondroitin Sulfate A/C, Dermatan Sulfate, Heparan Sulfate, Heparin, and Keratan Sulfate). Only clones that show exclusive binding to non-sulfated HA are selected for development.
Custom Anti-GAG Monoclonal Antibody Engineering
Successful binders (scFv/Fab) are converted into full-length immunoglobulins (IgG1, IgG4, or IgM) using recombinant expression in mammalian cells (HEK293 or CHO). We can engineer the Fc region to modulate effector functions or create multivalent formats (e.g., IgM) to increase avidity, which is often beneficial for binding repetitive carbohydrate epitopes like HMW-HA.
Key Features and Highlights
Non-Sulfated Specificity
Guaranteed lack of cross-reactivity with Chondroitin, Heparan, or Dermatan sulfates.
Size Discrimination
Ability to develop antibodies targeting specific HA oligosaccharide lengths (LMW-HA).
Recombinant Stability
Batch-to-batch consistency superior to animal-derived HA Binding Proteins (HABP).
Expert Project Support
PhD-level project management for complex GAG antibody projects.
Service Workflow: A Non-Immunization Approach
Start Your Anti-HA Project
Published Data
Hyaluronic acid (HA) accumulation in the tumor microenvironment (TME) creates a physical barrier that hinders drug delivery and immune cell infiltration. In a 2025 study published in Cancers, researchers utilized immunohistochemistry (IHC) to visualize the dense HA stroma in colorectal cancer (CRC) xenografts. As shown in Figure 1, the tumor tissue exhibits significant HA deposition (blue staining), which envelopes tumor cells. This accumulation is associated with aggressive tumor growth and resistance to therapy. The development of high-affinity anti-hyaluronic acid antibodies for tissue staining is crucial for characterizing this stromal barrier and evaluating HA-targeting therapeutics.
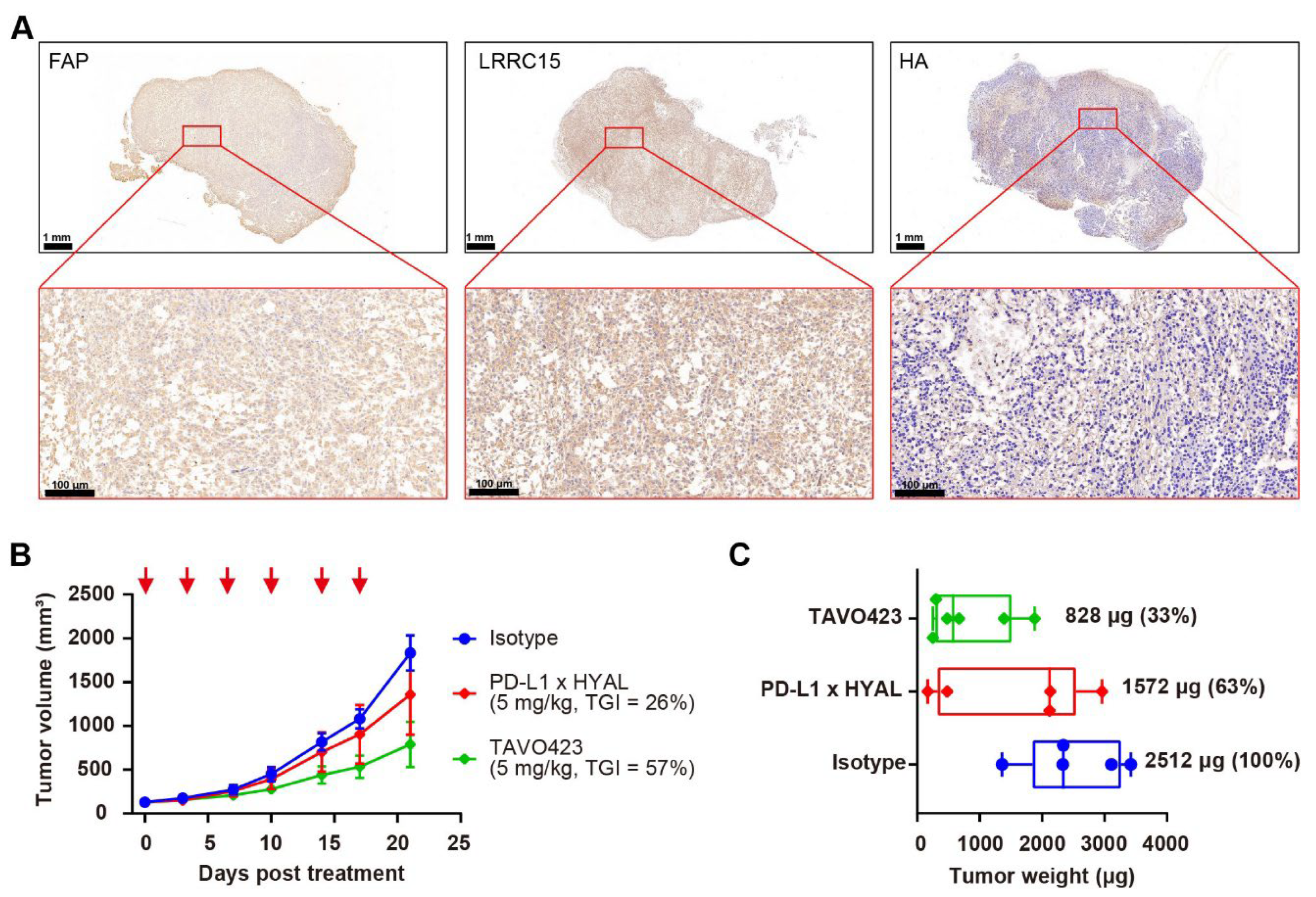 Fig.1 Representative immunohistochemistry analysis of hyaluronic acid expression in colorectal cancer xenografts.1
Fig.1 Representative immunohistochemistry analysis of hyaluronic acid expression in colorectal cancer xenografts.1
FAQs
Why is it so difficult to generate antibodies against Hyaluronic Acid?
HA is non-immunogenic because it is chemically identical across all mammals (100% homology), causing the host immune system to recognize it as "self." Additionally, its high water solubility and simple repeating structure make it difficult for immune cells to process and present. We solve this by using phage display technology, which bypasses the animal immune system entirely.
Can your antibodies distinguish between HA and Chondroitin Sulfate?
Yes. We include a rigorous counter-screening step in our panning process. We deplete the library of binders that recognize Chondroitin Sulfate (CS) or other GAGs, ensuring that the final anti-GAG antibody clones are specific only to the non-sulfated HA backbone.
Do you offer antibodies specific for HA size (LMW-HA vs HMW-HA)?
This is a challenging but possible target. By using defined HA oligosaccharides (e.g., HA4-HA10) as antigens, we can select for antibodies that recognize the specific conformation of short chains (LMW-HA) versus the entangled networks of HMW-HA. We can discuss the feasibility based on your specific needs.
What is the difference between an anti-HA antibody and HABP?
Hyaluronic Acid Binding Protein (HABP) is a natural protein fragment often used for staining. While effective, it can suffer from batch variability and broad binding. A monoclonal anti-hyaluronic acid antibody offers a chemically defined, reproducible reagent with potentially higher affinity and the ability to be engineered into various formats (e.g., IgG, Fab).
Reference:
- Zhou, Fulai, et al. "Targeted Hyaluronan Degradation Enhanced Tumor Growth Inhibition in Gastrointestinal Cancer Models." Cancers (2025): 17(21), 3411. Distributed under Open Access license CC BY 4.0. https://doi.org/10.3390/cancers17213411
Supports
- Anti-Heparan Sulfate (HS) Antibody Development Service
- Anti-Chondroitin Sulfate (CS) Antibody Development Service
- Anti-Dermatan Sulfate (DS) Antibody Development Service
- Anti-Keratan Sulfate (KS) Antibody Development Service
- Anti-Hyaluronic Acid (HA) Antibody Development Service
- Anti-GAG Sulfation Motif (Neo-epitope) Antibody Development Service
- Tumor-Associated GAG Antibody Development Service
